Radioactive contamination


Radioactive contamination, also called radiological contamination, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids or gases (including the human body), where their presence is unintended or undesirable (from IAEA definition).[6]
Such contamination presents a hazard because of the radioactive decay of the contaminants, which emit harmful ionising radiation such as alpha or beta particles, gamma rays or neutrons. The degree of hazard is determined by the concentration of the contaminants, the energy of the radiation being emitted, the type of radiation, and the proximity of the contamination to organs of the body. It is important to be clear that the contamination gives rise to the radiation hazard, and the terms "radiation" and "contamination" are not interchangeable.
Contamination may affect a person, a place, an animal, or an object such as clothing. Following an atmospheric nuclear weapon discharge or a nuclear reactor containment breach, the air, soil, people, plants, and animals in the vicinity will become contaminated by nuclear fuel and fission products. A spilled vial of radioactive material like Uranyl nitrate may contaminate the floor and any rags used to wipe up the spill. Cases of widespread radioactive contamination include the Bikini Atoll, the Rocky Flats Plant in Colorado, the Fukushima Daiichi nuclear disaster, the Chernobyl disaster, and the area around the Mayak facility in Russia.
Cleaning up contamination results in radioactive waste unless the radioactive material can be returned to commercial use by reprocessing. In some cases of large areas of contamination, the contamination may be mitigated by burying and covering the contaminated substances with concrete, soil, or rock to prevent further spread of the contamination to the environment. If a person's body is contaminated by ingestion or by injury and standard cleaning cannot reduce the contamination further, then the person may be permanently contaminated.
Some of the largest areas committed to be decontaminated are in the Fukushima Prefecture, Japan. The national government is under pressure to clean up radioactivity due to the Fukushima nuclear accident of March 2011 from as much land as possible so that some of the 110,000 displaced people can return. Stripping out the key radioisotope threatening health (caesium-137) from low level waste could also dramatically decrease the volume of waste requiring special disposal. A goal is to find techniques that might be able to strip out 80 to 95% of the caesium from contaminated soil and other materials, efficiently and without destroying the organic content in the soil. One being investigated is termed hydrothermal blasting. The caesium is broken away from soil particles and then precipitated with ferric ferricyanide (Prussian blue). It would be the only component of the waste requiring special burial sites. [7] The aim is to get annual exposure from the contaminated environment down to 1 millisievert (mSv) above background. The most contaminated area where radiation doses are greater than 50 mSv/year must remain off limits but some areas that are currently <5mSv/year may be decontaminated allowing 22,000 residents to return.
Sources of contamination
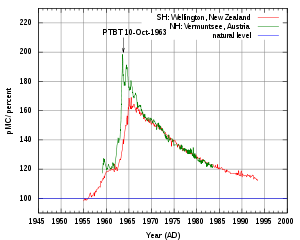
Radioactive contamination is typically the result of a spill or accident during the production, or use of, radionuclides (radioisotopes); these have unstable nuclei which are subject to radioactive decay.
Less typically, nuclear fallout is the distribution of radioactive contamination by a nuclear explosion. The amount of radioactive material released in an accident is called the source term.
Contamination may occur from radioactive gases, liquids or particles. For example, if a radionuclide used in nuclear medicine is spilled (accidentally or, as in the case of the Goiânia incident, through ignorance), the material could be spread by people as they walk around. Radioactive contamination may also be an inevitable result of certain processes, such as the release of radioactive xenon in nuclear fuel reprocessing. In cases that radioactive material cannot be contained, it may be diluted to safe concentrations. For a discussion of environmental contamination by alpha emitters please see actinides in the environment.
Contamination does not include residual radioactive material remaining at a site after the completion of decommissioning. Therefore, radioactive material in sealed and designated containers is not properly referred to as contamination, although the units of measurement might be the same.
Containment

Being within the intended "Containment" differentiates radioactive material from radioactive contamination. When radioactive materials are concentrated to a detectable level outside a planned containment, the area affected is generally referred to as "contaminated".
There are a large number of techniques for containing radioactive material so that it does not spread beyond the containment and become contamination. In the case of liquids this is by the use of high integrity tanks or containers, usually with a sump system so that leakage can be detected by radiometric or conventional instrumentation.
Where material is likely to become airborne, then extensive use is made of the glovebox, which is a common technique in hazardous laboratory and process operations in many industries. The gloveboxes are kept under a slight negative pressure and the vent gas is filtered in high efficiency filters, which are monitored by radiometric instrumentation to ensure they are functioning correctly.
Contamination accumulation
Radioactive contamination may exist on surfaces or in volumes of material or air.
Surface contamination
Surface contamination is usually expressed in units of radioactivity per unit of area. For SI, this is becquerels per square meter (or Bq/m²). Other units such as picoCuries per 100 cm² or disintegrations per minute per square centimeter (1 dpm/cm² = 167 Bq/m²) may be used. Surface contamination may either be fixed or removable. In the case of fixed contamination, the radioactive material cannot by definition be spread, but it is still measurable.
Detection and measurement of surface contamination of personnel and plant is normally by Geiger counter, scintillation counter or proportional counter. Proportional counters and dual phosphor scintillation counters can discriminate between alpha and beta contamination, but the Geiger counter cannot. Scintillation detectors are generally preferred for hand held monitoring instruments, and are designed with a large detection window. Geiger detectors tend to have small windows, but are more robust.
In the United Kingdom the HSE has issued a user guidance note on selecting the correct radiation measurement instrument for the application concerned . This covers all radiation instrument technologies, and is a useful comparative guide.
The spread of contamination by personnel exiting controlled areas in which nuclear material is used or processed is controlled by a variety of barrier techniques, sometimes involving changes of clothing and foot wear as required. Radiological instrumentation plays a key role in monitoring and detecting any potential contamination spread, and a combination of hand held survey instruments and permanently installed contamination monitors is used. The UK NPL publishes a guide on the alarm levels to be used with instruments for checking personnel exiting controlled areas in which contamination may be encountered.[8]
Airborne contamination
The air can be contaminated with radioactive isotopes in particulate form, which poses a particular inhalation hazard. Respirators with suitable air filters, or completely self-contained suits with their own air supply can mitigate these dangers.
Airborne contamination is measured by specialist radiometric instruments that continuously pump the sampled air through a filter. Airborne particles accumulate on the filter and can be measured in a number of ways:
1. The filter paper is periodically manually removed to an instrument which measures any accumulated radiation.
2. The filter paper is static and is measured in situ by a radiation detector
3. The filter is a slowly moving strip and is measured by a radiation detector. These are commonly called "moving filter" devices and automatically advance the filter to present a clean area for accumulation, and thereby allow a plot of airborne concentration over time.
Commonly a semiconductor radiation detection sensor is used that can also provide spectrographic information on the contamination being collected.
See the article on Airborne particulate radioactivity monitoring for more information.
Internal human contamination
Radioactive contamination can enter the body through ingestion, inhalation, absorption, or injection. For this reason, it is important to use personal protective equipment when working with radioactive materials. Radioactive contamination may also be ingested as the result of eating contaminated plants and animals or drinking contaminated water or milk from exposed animals. Following a major contamination incident, all potential pathways of internal exposure should be considered.
Contamination levels

Low level contamination
The hazards to people and the environment from radioactive contamination depend on the nature of the radioactive contaminant, the level of contamination, and the extent of the spread of contamination. Low levels of radioactive contamination pose little risk, but can still be detected by radiation instrumentation. If a survey or map is made of a contaminated area, random sampling locations may be labeled with their activity in becquerels or curies on contact. Low levels may be reported in counts per minute using a scintillation counter.
In the case of low-level contamination by isotopes with a short half-life, the best course of action may be to simply allow the material to naturally decay. Longer-lived isotopes should be cleaned up and properly disposed of, because even a very low level of radiation can be life-threatening when in long exposure to it.
Facilities and physical locations that are deemed to be contaminated may be cordoned off by a health physicist and labeled "Contaminated area." Persons coming near such an area would typically require anti-contamination clothing or anti-c's.
High level contamination
High levels of contamination may pose major risks to people and the environment. People can be exposed to potentially lethal radiation levels, both externally and internally, from the spread of contamination following an accident (or a deliberate initiation) involving large quantities of radioactive material. The biological effects of external exposure to radioactive contamination are generally the same as those from an external radiation source not involving radioactive materials, such as x-ray machines, and are dependent on the absorbed dose.
When radioactive contamination is being measured or mapped in situ, any location that appears to be a point source of radiation is likely to be heavily contaminated. A highly contaminated location is colloquially referred to as a "hot spot." On a map of a contaminated place, hot spots may be labeled with their "on contact" dose rate in mSv/hr. In a contaminated facility, hot spots may be marked with a sign, shielded with bags of lead shot, or cordoned off with warning tape containing the radioactive trefoil symbol in magenta on a yellow background.
Radiation hazards

The hazard from contamination is the emission of ionising radiation. The principal radiations which will be encountered are alpha, beta and gamma, but these have quite different characteristics. They have widely differing penetrating powers and radiation effect, and the accompanying diagram shows the penetration of these radiations in simple terms. For an understanding of the different ionising effects of these radiations and the weighting factors applied, see the article on absorbed dose.
Radiation monitoring involves the measurement of radiation dose or radionuclide contamination for reasons related to the assessment or control of exposure to radiation or radioactive substances, and the interpretation of the results. The methodological and technical details of the design and operation of environmental radiation monitoring programmes and systems for different radionuclides, environmental media and types of facility are given in IAEA Safety Standards Series No. RS–G-1.8[9] and in IAEA Safety Reports Series No. 64.[10]
Background radiation
In the natural world, there is always radiation emitted from radionuclides as they decay. Not only is the entire world constantly bombarded by cosmic rays, but practically every living thing on earth contains carbon-14 and tritium and most (including humans) contain some potassium-40. These levels of radioactivity pose little danger but can confuse measurement. A particular problem is encountered with naturally generated Radon gas which can affect instruments which are set to detect contamination close to normal background levels and can cause false alarms. Because of this skill is required by the operator of radiological survey equipment to differentiate between background radiation and the radiation which emanates from contamination.
Effects of contamination
Biological effects
The biological effects of internally deposited radionuclides depend greatly on the activity, the biodistribution, and the removal rates of the radionuclide, which in turn depends on its chemical form, the particle size, and route of entry. Effects may also depend on the chemical toxicity of the deposited material, independent of its radioactivity. Some radionuclides may be generally distributed throughout the body and rapidly removed, as is the case with tritiated water.
Some organs concentrate certain elements and hence radionuclide variants of those elements. This action may lead to much lower removal rates. For instance, the thyroid gland takes up a large percentage of any iodine that enters the body. Large quantities of inhaled or ingested radioactive iodine may impair or destroy the thyroid, while other tissues are affected to a lesser extent. Radioactive iodine-131 is a common fission product; it was a major component of the radiation released from the Chernobyl disaster, leading to nine fatal cases of pediatric thyroid cancer and hypothyroidism. On the other hand, radioactive iodine is used in the diagnosis and treatment of many diseases of the thyroid precisely because of the thyroid's selective uptake of iodine.
Mental health effects
The consequences of low-level radiation are often more psychological than radiological. Because damage from very-low-level radiation cannot be detected, people exposed to it are left in anguished uncertainty about what will happen to them. Many believe they have been fundamentally contaminated for life and may refuse to have children for fear of birth defects. They may be shunned by others in their community who fear a sort of mysterious contagion.[11]
Forced evacuation from a radiation or nuclear accident may lead to social isolation, anxiety, depression, psychosomatic medical problems, reckless behavior, even suicide. Such was the outcome of the 1986 Chernobyl nuclear disaster in the Ukraine. A comprehensive 2005 study concluded that "the mental health impact of Chernobyl is the largest public health problem unleashed by the accident to date".[11] Frank N. von Hippel, a U.S. scientist, commented on the 2011 Fukushima nuclear disaster, saying that "fear of ionizing radiation could have long-term psychological effects on a large portion of the population in the contaminated areas".[12]
Such great psychological danger does not accompany other materials that put people at risk of cancer and other deadly illness. Visceral fear is not widely aroused by, for example, the daily emissions from coal burning, although, as a National Academy of Sciences study found, this causes 10,000 premature deaths a year in the US population of 317,413,000. Medical errors leading to death in U.S. hospitals are estimated to be between 44,000 and 98,000. It is "only nuclear radiation that bears a huge psychological burden — for it carries a unique historical legacy".[11]
In the media
- The Atom Strikes! (1945) — official US War Department film documenting damage to Hiroshima and Nagasaki.
- Gembaku no ko (1952) — documentary showing a Japanese school teacher who visits her hometown of Hiroshima 6 years after the bombing to find the horrors of radiation.
- The War Game (1965) - banned television docudrama about a Soviet nuclear attack on Britain, not shown on TV until 1985
- Hiroshima Nagasaki August 1945 (1970) — documentary of atomic bomb devastation.
- The Atomic Cafe (1982) — documentary combines stock US government footage of nuclear testing along with propaganda films shown in public schools in the 1940s and 1950s about how citizens should respond to atomic attacks.
- The Day After (1983) — TV docudrama about the effects of a nuclear holocaust on the small-town residents in eastern Kansas.
- Threads (1984) — British television drama featuring a nuclear war and its effects on the city of Sheffield in northern England.
- Radio Bikini (1988) — documentary film about Bikini Atoll atomic tests. Gruesome details and pictures of joking sailors being irradiated, and interview with an injured (irradiated) sailor.
- Hiroshima: Out of the Ashes (1990) — TV history and graphic depictions of the horror of nuclear war.
- K-19: Doomsday Submarine (2002) — TV documentary about Russia's disastrous first nuclear submarine.
- K-19: The Widowmaker (2002) — docudrama about the first of many disasters that befell the Soviet submarine K-19
- The Last Atomic Bomb (2006) — documentary about the fate of the survivors of Nagasaki 1945.
- White Light/Black Rain: The Destruction of Hiroshima and Nagasaki (2007) — HBO documentary showing how many teenage Japanese are ignorant of what happened in 1945. Also includes some American atomic veterans.
Also see Criticality accident for six more films about radioactive issues.
See also
|
- Background radiation
- Bikini Atoll
- Chemical contamination
- Chernobyl disaster
- Criticality accident
- Goiânia accident
- Human decontamination
- Ionizing radiation (includes physicists' units of radiation exposure)
- List of Milestone nuclear explosions
- Lists of nuclear disasters and radioactive incidents
- Low-background steel
- Mayak – Russian facility which has caused many deaths in the surrounding area
- Nuclear and radiation accidents
- Nuclear debate (disambiguation)
- Nuclear power
- Radiation biology
- Radiation exposure (disambiguation)
- Radiation poisoning
- Radioactive contamination from the Rocky Flats Plant
- Radioactive waste
- Radiophobia
- Relative Biological Effectiveness
- Rongelap Atoll
- Soviet submarine K-19
References
- ↑ Richard Schiffman (12 March 2013). "Two years on, America hasn't learned lessons of Fukushima nuclear disaster". The Guardian.
- ↑ Martin Fackler (June 1, 2011). "Report Finds Japan Underestimated Tsunami Danger". New York Times.
- ↑ "Atmospheric δ14C record from Wellington". Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center (Oak Ridge National Laboratory). 1994. Retrieved 2007-06-11.
- ↑ Levin, I., et al. (1994). "δ14C record from Vermunt". Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center.
- ↑ "Radiocarbon dating". University of Utrecht. Retrieved 2008-02-19.
- ↑ International Atomic Energy Agency (2007). IAEA Safety Glossary: Terminology Used in Nuclear Safety and Radiation Protection. Vienna: IAEA. ISBN 92-0-100707-8.
- ↑ Dennis Normile, "Cooling a Hot Zone," Science, 339 (1 March 2013) pp. 1028-1029.
- ↑ Operational Monitoring Good Practice Guide "The Selection of Alarm Levels for Personnel Exit Monitors" Dec 2009 - National Physical Laboratory, Teddington UK
- ↑ International Atomic Energy Agency (2005). Environmental and Source Monitoring for Purposes of Radiation Protection, IAEA Safety Standards Series No. RS–G-1.8. Vienna: IAEA.
- ↑ International Atomic Energy Agency (2010). Programmes and Systems for Source and Environmental Radiation Monitoring. Safety Reports Series No. 64.. Vienna: IAEA. p. 234. ISBN 978-92-0-112409-8.
- ↑ 11.0 11.1 11.2 Andrew C. Revkin (March 10, 2012). "Nuclear Risk and Fear, from Hiroshima to Fukushima". New York Times.
- ↑ Frank N. von Hippel (September/October 2011 vol. 67 no. 5). "The radiological and psychological consequences of the Fukushima Daiichi accident". Bulletin of the Atomic Scientists. pp. 27–36.
- Measurement Good Practice Guide No. 30 "Practical Radiation Monitoring" Oct 2002 - National Physical Laboratory, Teddington UK
External links
- Alliance for Nuclear Responsibility
- training guide Brookhaven National Laboratory Training Guide.
