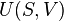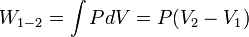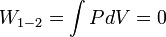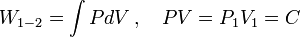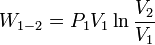Quasistatic process
| Thermodynamics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
|
Branches |
||||||||||||||
|
History / Culture
|
||||||||||||||
In thermodynamics, a quasistatic process is a thermodynamic process that happens infinitely slowly. No real process is quasistatic, but such processes can be approximated by performing them very slowly.
Any reversible process is necessarily a quasistatic one. However, some quasistatic processes are irreversible, if there is heat flowing (in or out of the system) or if entropy is being created in some other way. An example of a quasistatic process that is not reversible is a compression against a system with a piston subject to friction — although the system is always in thermal equilibrium, the friction ensures the generation of dissipative entropy, which directly goes against the definition of reversible. A notable example of a process that is not even quasistatic is the slow heat exchange between two bodies at two finitely different temperatures, where the heat exchange rate is controlled by an approximately adiabatic partition between the two bodies — in this case, no matter how slowly the process takes place, the states of the two bodies are never infinitesimally close to equilibrium, since thermal equilibrium requires that the two bodies be at precisely the same temperature.
Some ambiguity exists in the literature concerning the distinction between quasistatic and reversible processes, as these are sometimes taken as synonyms. The reason is precisely because of the proven theorem that any reversible process is also a quasistatic one, even though we have also shown that the converse is not true. It is practically not useful to differentiate between the two because any engineer would remember to include friction when calculating the dissipative entropy generation. The above definition is closer to the intuitive understanding of the word “quasi-” (almost) “static”, while remaining technically different from reversible processes.
PV-Work in various quasi-static processes
- Constant pressure: Isobaric processes,
- Constant volume: Isochoric processes,
- Constant temperature: Isothermal processes,
- Polytropic processes,
Bibliography
- Sears, F.W., Salinger, G.L. (1986), Thermodynamics, Kinetic Theory, and Statistical Thermodynamics, 3rd edition (Addison-Wesley)
- Lavenda, B.H. (1978), Thermodynamics of Irreversible Processes, Halsted.
- Colin finn 'thermal physics'
- M C Sprackling 'thermal physics'
- P.K. Nag 'Engineering Thermodynamics'