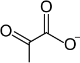
Pyruvate decarboxylation
Pyruvate decarboxylation (also known as the Swanson Conversion,[1] oxidative decarboxylation reaction or link reaction) is the far from equilibrium biochemical reaction that uses pyruvate to form acetyl-CoA, releasing NADH, a reducing equivalent, and carbon dioxide via decarboxylation. It is known as the link reaction because it forms an important link between the metabolic pathways of glycolysis and the citric acid cycle. This reaction is usually catalyzed by the pyruvate dehydrogenase complex as part of aerobic respiration.[2] In eukaryotes, pyruvate decarboxylation takes place exclusively inside the mitochondrial matrix; in prokaryotes similar reactions take place in the cytoplasm and at the plasma membrane.[3]
| ||||||||||||||||||||
This reaction is very common in most organisms as a link to the citric acid cycle. This reaction is carried out in the mitochondria, unlike the reactions of glycolysis which are cytosolic.
The conversion of pyruvate to acetyl CoA by the pyruvate dehydrogenase complex is a key step in the liver in particular, as it removes any chance of conversion of pyruvate to glucose, or as a transamination substrate. It commits pyruvate to entering the citric acid cycle, where it is either used as a substrate for oxidative phosphorylation, or is converted to citrate for export to the cytosol to serve as a substrate for fatty acid and isoprenoid biosynthesis.
The oxidative decarboxylation of pyruvate in anaerobic organisms differs from the aerobic process in that the electron acceptor is an iron-sulfur protein, not NAD+. The conversion is catalyzed by a thiamine-dependent enzyme that also acylates coenzyme A.[4] The reducing equivalents are disposed of by the production of H2 via hydrogenase.
To summarise:
1. Pyruvate is decarboxylated
2. It is added to CoA to form Acetyl CoA
acetyl CoA is then ready for use in the Krebs cycle.

References
- ↑ 1
- ↑ Alberts et al. Molecular Biology of the Cell. Garland Science, 2001. ISBN 0-8153-4072-9
- ↑ Raven et al. Biology, 8th edition. McGraw Hill, 2008. ISBN 978-0-07-110202-5
- ↑ Eric Chabrière, Xavier Vernède, Bruno Guigliarelli, Marie-Hélène Charon, E. Claude Hatchikian, Juan C. Fontecilla-Camps “Crystal Structure of the Free Radical Intermediate of Pyruvate:Ferredoxin Oxidoreductase” Science 2001, Volume 294, page 2559.
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||