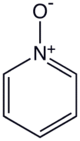
Pyridine-N-oxide
| Pyridine-N-oxide | |
|---|---|
 |
 |
| IUPAC name Pyridine-N-oxide | |
| Other names pyridine-1-oxide | |
| Identifiers | |
| CAS number | 694-59-7 |
| PubChem | 12753 |
| ChemSpider | 12229 |
| ChEBI | CHEBI:29136 |
| Jmol-3D images | {{#if:c1cc[n+](cc1)[O-]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C5H5NO |
| Molar mass | 95.10 g mol−1 |
| Appearance | colourless solid |
| Melting point | 65–66 °C |
| Boiling point | 270 °C; 518 °F; 543 K |
| Solubility in water | high |
| Acidity (pKa) | 0.8 (of conjugate acid) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peracids as the oxidising agent.[1] The molecule is planar. The compound is used infrequently as an oxidizing reagent in organic synthesis.[2] It also serves as a ligand in coordination chemistry.
Synthesis
The oxidation of pyridine is effected with peracetic acid, a reaction that affords the protonated derivative. Subsequent treatment with base liberates the neutral oxide.[3]
Safety
The compound is a skin irritant.
References
- ↑ J. Meisenheimer (1926). "Über Pyridin-, Chinolin- und Isochinolin-N-oxyd". Berichte der deutschen chemischen Gesellschaft 59 (8): 1848–1853. doi:10.1002/cber.19260590828.
- ↑ S. Nicholas Kilényi "Pyridine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley & Sons, New York. doi:10.1002/047084289X.rp283 Article Online Posting Date: April 15, 2001
- ↑ H. S. Mosher, L. Turner, and A. Carlsmith (1963), "Pyridine-N-oxide", Org. Synth.; Coll. Vol. 4: 828