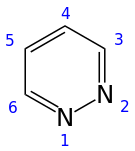
Pyridazine
| Pyridazine | |
|---|---|
 | |
 |
 |
| IUPAC name Pyridazine | |
| Other names 1,2-diazine, orthodiazine, oizine | |
| Identifiers | |
| CAS number | 289-80-5 |
| PubChem | 9259 |
| ChemSpider | 8902 |
| ChEBI | CHEBI:30954 |
| ChEMBL | CHEMBL15719 |
| RTECS number | GY2390000 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C4H4N2 |
| Molar mass | 80.09 g mol−1 |
| Appearance | colorless liquid |
| Density | 1.107 g/cm3 |
| Melting point | −8 °C; 18 °F; 265 K |
| Boiling point | 208 °C; 406 °F; 481 K |
| Related compounds | |
| Related compounds | pyridine, pyrimidine, pyrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Pyridazine is a heterocyclic organic compound with the molecular formula (CH)4N2. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other (CH)4N2 rings, pyrimidine and pyrazine.
Occurrence
Pyridazines are rare in nature, possibly reflecting the scarcity of naturally occurring hydrazines, common building blocks for the synthesis of these heterocycles. The pyridazine structure is a popular pharmacophore. It found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found within the structure of several drugs such as cefozopran, cadralazine, minaprine, pipofezine, hydralazine, and cilazapril.
Syntheses
In the course of his classic investigation on the Fischer indole synthesis, Emil Fischer prepared the first pyridazine via the condensation of phenylhydrazine and levulinic acid.[1] The parent heterocycle was first prepared by oxidation of benzocinnoline to the pyridazinetetracarboxylic acid followed by decarboxylation. A better route to this otherwise esoteric compound starts with the maleic hydrazide. These heterocycles are often prepared via condensation of 1,4-diketones or 4-ketoacids with hydrazines.[2]
References
- ↑ E. Fischer "Indole aus Phenylhydrazin" Justus Liebigs Annalen der Chemie 1886, Volume 236, Issue 1-2, pages 126–151. doi:10.1002/jlac.18862360107
- ↑ M. Tišler, B. Stanovnik "Pyridazines" Advances in Heterocyclic Chemistry, Volume 9, 1968, Pages 211-320. http://dx.doi.org/10.1016/S0065-2725(08)60374-8