Psoralen
| Psoralen | |
|---|---|
 | |
| IUPAC name 7H-furo[3,2-g]chromen-7-one | |
| Other names 7H-furo[3,2-g][1]benzopyran-7-one | |
| Identifiers | |
| CAS number | 66-97-7 |
| ChemSpider | 5964 |
| ChEBI | CHEBI:27616 |
| ChEMBL | CHEMBL164660 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C11H6O3 |
| Molar mass | 186.16 g/mol |
| Melting point | 158–161 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Psoralen (also called psoralene) is the parent compound in a family of natural products known as furocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley and West Indian satinwood. It is widely used in PUVA (= psoralen + UVA) treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma. Many furocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.
Uses
An important use of psoralen is in PUVA treatment for skin problems such as psoriasis and (to a lesser extent) eczema and vitiligo. This takes advantage of the high UV absorbance of psoralen. The psoralen is applied first to sensitise the skin, then UVA light is applied to clean up the skin problem. Psoralen has also been recommended for treating alopecia.[citation needed] Psoralens are also used in photopheresis, where they are mixed with the extracted leukocytes before UV radiation is applied.
Psoralen is a mutagen, and is used for this purpose in molecular biology research. Psoralen intercalates into the DNA and, on exposure to ultraviolet (UVA) radiation, can form covalent interstrand cross-links (ICL) with thymines preferentially at 5'-TpA sites in the genome, inducing apoptosis. Psoralen plus UVA (PUVA) therapy has shown considerable clinical efficacy.[1] Unfortunately, a side effect of PUVA treatment is a higher risk of skin cancer.[2]
Despite the photocarcinogenic properties of psoralen,[3][4] It had been used as a tanning activator in sunscreens until 1996.[5] Psoralens are used in tanning accelerators, but users should keep in mind that psoralen increases the skin’s sensitivity to light. Some patients have had severe skin loss after sunbathing with psoralen-containing tanning activators.[6] Patients with lighter skin colour suffer four times as much from the melanoma-generating properties of psoralens than those with darker skin[5] The synthetic amino-psoralen, amotosalen HCl, has been developed for the inactivation of infectious pathogens (bacteria, viruses, protozoa) in platelet and plasma blood components prepared for transfusion support of patients. The technology is currently in routine use in certain European blood centers.[7][8]
Chemistry
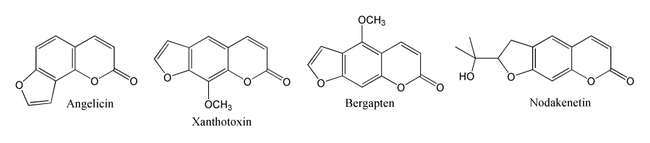
One isomer of psoralen is angelicin, and most furocoumarins can be regarded as derivatives of psoralen or angelicin. Some important psoralen derivatives are Imperatorin, xanthotoxin, bergapten and nodekenetin.
Another important feature of this class of compounds is their ability to generate singlet oxygen.
Structure
The structure of psoralen was originally deduced by identifying the products of its degradation reactions. It exhibits the normal reactions of the lactone of coumarin, such as ring opening by alkali to give a coumarinic acid or coumaric acid derivative. Potassium permanganate causes oxidation of the furan ring, while other methods of oxidation produce furan-2,3-carboxylic acid.
Synthesis
Psoralen synthesis is difficult, due the fact that umbelliferone undergoes substitution at the 8-position rather than at the desired 6 position. Benzofuran reacts preferentially in the furan ring rather than in the benzene ring. However, the 7-hydroxy derivative of 2,3-dihydrobenzofuran (also called coumaran) does undergo substitution at the desired 6-position allowing the following synthesis of the coumarin system via a Gattermann-Koch reaction followed by a Perkin condensation using acetic anhydride. The synthesis is then completed by dehydrogenation of the five-membered ring to produce the furan ring.

Biosynthesis
Psoralen originates from coumarins in the shikimate pathway; its biosynthesis is shown in the figure below. The aromatic ring in 6 is activated at positions ortho to the hydroxyl group, and is alkylated by 5, an alkylating agent. The dimethylallyl group in 7 then undergoes cyclization with the phenol group to give 8. This transformation is catalysed by a cytochome P-450-dependent monooxygenase17 (psoralen 5-monooxygenase), and cofactors (NADPH) and molecular oxygen.[9]
A biosynthetic pathway in which psoralen is formed is shown in the figure below. A second P-450-dependent monooxygenase enzyme (psoralen synthase) then cleaves off 10 (in the form of 11) from 8 to give 1. This pathway does not involve any hydroxylated intermediate, and cleavage is postulated to be initiated by a radical reaction.[9]
Plant sources
Ficus carica (fig) is probably the most abundant source of psoralens. They are also found in small quantities in Ammi visnaga (bisnaga), Pastinaca sativa (parsnip), Petroselinum crispum (parsley), Levisticum officinale (lovage), Foeniculum vulgare (fruit, i.e., fennel seeds), Daucus carota (carrot), Psoralea corylifolia (babchi), and Apium graveolens (celery).[10]
References
- ↑ Wu Q, Christensen LA, Legerski RJ, Vasquez KM (June 2005). "Mismatch repair participates in error-free processing of DNA interstrand crosslinks in human cells". EMBO Rep. 6 (6): 551–7. doi:10.1038/sj.embor.7400418. PMC 1369090. PMID 15891767.
- ↑ Momtaz K, Fitzpatrick TB (April 1998). "The benefits and risks of long-term PUVA photochemotherapy". Dermatol Clin 16 (2): 227–34. doi:10.1016/S0733-8635(05)70005-X. PMID 9589196.
- ↑ M. J. Ashwood-Smith; G. A. Poulton; M. Barker; M. Mildenberger E (1980). "5-Methoxypsoralen, an ingredient in several suntan preparations, has lethal, mutagenic and clastogenic properties". Nature 285 (5): 407–9. doi:10.1038/285407a0. PMID 6991953.
- ↑ Zajdela F, Bisagni E. (1981). "5-Methoxypsoralen, the melanogenic additive in suntan preparations, is tumorigenic in mice exposed to 365 nm UV radiation". Carcinogenesis 1981 (2): 121–7. doi:10.1093/carcin/2.2.121. PMID 7273295.
- ↑ 5.0 5.1 Autier P., Dore J.-F., Cesarini J.-P. (1997). "Should subjects who used psoralen suntan activators be screened for melanoma?". Annals of Oncology 8 (5): 435–7. doi:10.1023/A:1008205513771. PMID 9233521.
- ↑ Nettelblad H, Vahlqvist C, Krysander L, Sjöberg F (December 1996). "Psoralens used for cosmetic sun tanning: an unusual cause of extensive burn injury". Burns 22 (8): 633–5. doi:10.1016/S0305-4179(96)00028-9. PMID 8982544.
- ↑ Osselaer et al. (2009). "Universal adoption of pathogen inactivation of platelet components: impact on platelet and red blood cell component use". Transfusion 49: 1412.
- ↑ Cazenave et al. (2010). "An active hemovigilance program characterizing the safety profile of 7,483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment". Transfusion 50: 1210.
- ↑ 9.0 9.1 Dewick, P.M. (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). Wiley. pp. 164–5. ISBN 0-471-49641-3.
- ↑ Dr. Duke's Phytochemical and Ethnobotanical Databases (on line)
Further reading
- Dean, F.M. (1963). Naturally Occurring Oxygen Ring Compounds. London: Butterworths.
- The Merck Index (7th ed.). Rahway NJ: Merck. 1960.
External links
| Wikimedia Commons has media related to Psoralen. |
| |||||||||||||||||||||||||||||||