2C-T-4
| 2C-T-4 | |
|---|---|
 | |
 | |
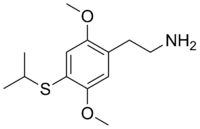
| 2-[4-(Isopropylthio)-2,5-dimethoxyphenyl]ethanamine | |
| Identifiers | |
| CAS number | 207740-25-8 |
| ChemSpider | 21106232 |
| ChEMBL | CHEMBL338259 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C13H21NO2S |
| Molar mass | 255.38 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
2C-T-4 or 2,5-dimethoxy-4-isopropylthiophenethylamine is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen.
Chemistry
2C-T-4 is the 2-carbon homologue of Aleph-4. The full chemical name is 2-4-(isopropylthio)-2,5-dimethoxyphenylethanamine. The drug has structural and pharmacodynamic properties similar to 2C-T-7 and 2C-T-9.
Dosage
2C-T-4 is usually taken orally, and the dosage range is typically 8-20 mg.
Effects
2C-T-4 produces psychedelic and entheogenic effects that develop slowly and can last 8–16 hours. Some users have also reported dissociative properties uncharacteristic of other psychedelic phenethylamines. While all users experience virtually no effects for the first hour after ingestion, results vary drastically between individuals and range from hallucination and euphoria to intense sickness and anxiety.[1] Shulgin devoted a chapter in the first part of PiHKAL to this compound, describing it as an intense "plus-four" psychedelic.
Pharmacology
The mechanism that produces 2C-T-4’s hallucinogenic and entheogenic effects has not been specifically established, however it is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines for which the mechanism of action is known.
Dangers
The toxicity of 2C-T-4 is not well documented. It may be expected that it would act in a manner similar to that of other phenethylamines, especially of the 2C-T family. Other phenethylamine derivatives substituted with an alkylthio group at the 4 position such as 2C-T-7 and 4-MTA are known to act as selective monoamine oxidase A inhibitors, a side effect which can lead to lethal serotonin syndrome when they are combined with stimulant drugs. Most confirmed fatalities involving 2C-T drugs involve their combination with other drugs such as alcohol or cocaine. To those already inebriated with alcohol, 2C-T-4 has a dangerous sobering effect that could lead chronically abusive or inexperienced users to drink until lethally poisoned. Based on the known toxicity of other drugs of this family, doses above 20 milligrams of 2C-T-4 may have a high risk of very unpleasant physiological experiences, and at doses of 30 mg or above, death from overdose might occur.
Popularity
2C-T-4 is relatively unknown on the black market, but has been sold to a limited extent on the research chemical market.
Drug prohibition laws
Denmark
2C-T-4 is added to the list of Schedule B controlled substances.[2]
Sweden
Sveriges riksdags health ministry Statens folkhälsoinstitut classified 2C-T-4 as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Jul 15, 2007, in their regulation SFS 2007:600 listed as 2,5-dimetoxi-4-isopropyltiofenetylamin (2C-T-4), making it illegal to sell or possess.[3]
USA
As of July 9, 2012, 2C-T-4 is a Schedule I substance in the United States, under the Synthetic Drug Abuse Prevention Act of 2012.[4]
Homologue
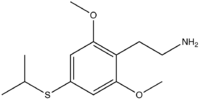
2C-T-4 has one homologue, the structural isomer Ψ-2C-T-4 (2,6-dimethoxy-4-(i)-propylthiophenethylamine). This compound was tested by Alexander Shulgin at a dose of 12 mg.
At this dosage its duration was very short and it produced few effects, however based on the research into the better characterized compound Ψ-DOM, the potency of Ψ-2C-T-4 is likely to be around 1/3 that of 2C-T-4 itself, so a more effective dosage of Ψ-2C-T-4 might be in the region of 20–60 mg;[1] however high doses such as this might well be associated with toxic side effects, and so extreme caution would be advised.
References
- ↑ 1.0 1.1 Shulgin, Alexander; Ann Shulgin (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ↑ https://www.retsinformation.dk/Forms/R0710.aspx?id=137169
- ↑ http://www.notisum.se/rnp/sls/sfs/20070600.pdf
- ↑ Portman. "Synthetic Drug Abuse Prevention Act of 2012". Govtrack. Retrieved 22 July 2012.
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||