Prunasin
| Prunasin | ||
|---|---|---|
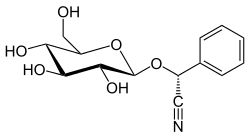 | ||
| IUPAC name (2R)-2-phenyl-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyacetonitrile | ||
| Other names (R)-Prunasin | ||
| Identifiers | ||
| CAS number | 99-18-3 | |
| PubChem | 119033 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C14H17NO6 | |
| Molar mass | 295.29 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Prunasin is a cyanogenic glucoside related to amygdalin.
Natural occurrences
Prunasin is found in species in the genus Prunus such as Prunus japonica or P. maximowiczii and in bitter almonds.[1] It is also found in leaves and stems of Olinia ventosa, O. radiata, O. emarginata and O. rochetiana[2] or in Acacia greggii.
It is also found in dandelion coffee, a coffee substitute.
Metabolism
Prunasin beta-glucosidase is an enzyme that uses (R)-prunasin and H2O to produce D-glucose and mandelonitrile.
Amygdalin beta-glucosidase is an enzyme that uses (R)-amygdalin and H2O to produce (R)-prunasin and D-glucose.
References
- ↑ Sanchez-Perez, R.; Belmonte, F. S.; Borch, J.; Dicenta, F.; Møller, B. L.; Jørgensen, K. (2012). "Prunasin Hydrolases during Fruit Development in Sweet and Bitter Almonds". Plant Physiology 158 (4): 1916–32. doi:10.1104/pp.111.192021. PMC 3320195. PMID 22353576.
- ↑ Nahrstedt, Adolf; Rockenbach, Jürgen (1993). "Occurrence of the cyanogenic glucoside prunasin and II corresponding mandelic acid amide glucoside in Olinia species (oliniaceae)". Phytochemistry 34 (2): 433. doi:10.1016/0031-9422(93)80024-M.