Propargyl bromide
| Propargyl bromide | |
|---|---|
 | |
| IUPAC name 3-Bromo-1-propyne | |
| Other names Bromopropyne, 1-Brom-2-propin, 1-Bromo-2-propyne Gamma-bromoallylene, 1-Bromo-2-propyne, 2-Propynyl bromide, Propargyl bromide, Propynyl bromide, gamma-Bromoallylene | |
| Identifiers | |
| CAS number | 106-96-7 |
| PubChem | 7842 |
| ChemSpider | 7554 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C3H3Br |
| Molar mass | 118.96 g mol−1 |
| Appearance | colourless liquid |
| Density | 1.57 g/mL (20 °C)[1] |
| Melting point | −61.1 °C; −78.0 °F; 212.1 K ([1]) |
| Boiling point | 89 °C; 192 °F; 362 K ([1]) |
| Solubility in water | Insoluble |
| Solubility | Soluble in organic solvents |
| log P | 1.179 |
| Vapor pressure | 72 mbar (20 °C)[1] |
| Hazards | |
| Main hazards | Highly Flammable, Toxic, Corrosive |
| NFPA 704 |
 3
3
4
|
| Flash point | 18 °C (64 °F)[1] |
| Autoignition temperature | 324 °C (615 °F)[1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Propargyl bromide, also known as 3-bromo-1-propyne, is an organic compound with the chemical formula CHCCH2Br. It is a halogenated organic compound consisting of an alkyl functional group with 2-propynyl group linked to bromide. It has a lachrymatory effect, like related compounds. The compound is a useful reagent in organic synthesis.
Applications
In the 1960s, propargyl bromide was first used in a soil fumigant called Trizone.[2]
Propargyl bromide can also be used as an intermediate for the synthesis of organic compounds, including agrochemicals and pharmaceuticals. It forms a Grignard reagent at low temperatures, for example.[3]
Production
Propargyl bromide may be produced by the treatment of propargyl alcohol with phosphorus tribromide:[4]
Reactions
Propargyl bromide can be used in enyne metathesis of propargylic amines, propargylation of spiro ketones, production of allylic alcohols, and enone complexes.[5]
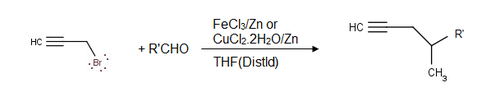
Aldehydes may be reacted with propargyl bromide in a Barbier-type reaction to yield alkyne alcohols as well:[6]
Safety
Propargyl bromide is a lachrymator and an alkylating agent.[7]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Record in the GESTIS Substance Database from the IFA
- ↑ Franz Müller and Arnold P. Applebyki "Weed Control, 2. Individual Herbicides" in Ullmann's Encyclopedia of Industrial Chemistry 2010 doi:10.1002/14356007.o28_o01
- ↑ Henning Hopf, Ingrid Böhm, and Jürgen Kleinschroth (1990), "Diels-Alder Reaction of 1,2,4,5-Hexatetraene: Tetramethyl[2.2]paracyclophane-4,5,12,13-tetracarboxylate", Org. Synth. 60: 41; Coll. Vol. 7: 485
- ↑ "Process for Producing Propargyl Bromide". Retrieved November 7, 2012.
- ↑ "Propargyl Bromide". Retrieved November 5, 2012.
- ↑ Ghosh, P.; Chattopadhyay, A. (2012). "A practical procedure of propargylation of aldehydes". Tet. Lett. 53 (39): 5202–5205. doi:10.1016/j.tetlet.2012.07.021.
- ↑ "3-Bromo-1-Propyne". Retrieved November 3, 2012.