Primary cell
A primary cell is a battery that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity and is useless. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants. Primary cells are made in a range of standard sizes to power small household appliances.
Comparison with rechargeables
Rechargeable batteries are economical to use when their initially higher cost and cost of a charging system can be spread out over many use cycles; for example, in hand-held power tools, it would be very costly to replace a high-capacity primary battery pack every few hours of use.
Primary batteries are useful where long periods of storage are required; a primary battery can be constructed to have a lower self-discharge rate than a rechargeable battery, so all its capacity is available for useful purposes. Applications that require a small current for a long time, for example a smoke detector, also use primary batteries since the self-discharge current of a rechargeable battery would exceed the load current and limit service time to a few days or weeks. For example, a flashlight used for emergencies must work when needed, even if it has sat on a shelf for a long time. Primary cells are also more cost-efficient in this case, as rechargeable batteries would use only a small fraction of available recharge cycles. Reserve batteries achieve very long storage time (on the order of 10 years or more) without loss of capacity, by physically separating the components of the battery and only assembling them at the time of use. Such constructions are expensive but are found in applications like munitions, which may be stored for years before use.
Recharging primary cells
Attempts to extend the life of primary cells, for example by recharging alkaline batteries, meet with variable results. Third-party devices are manufactured and represented as being able to recharge primary cells. The internal chemical reactions of a primary cell are not easily reversed by externally applied currents, so reactants do not entirely return to their initial state and location. Recharged primary cells will not have the life or performance of secondary cells. Primary cell manufacturers often warn against recharging.
Polarization
A major factor reducing the lifetime of primary cells is that they become polarized during use. This means that hydrogen accumulates at the cathode and reduces the effectiveness of the cell.
To reduce the effects of polarization in commercial cells to extend their lives, chemical depolarization is used; that is, an oxidizing agent is added to the cell, to oxidize the hydrogen to water. Manganese dioxide is used in the Leclanché cell and zinc–carbon cell, and nitric acid is used in the Bunsen cell and Grove cell.
Attempts have been made to make simple cells self-depolarizing by roughening the surface of the copper plate to facilitate the detachment of hydrogen bubbles with little success. Electrochemical depolarization exchanges the hydrogen for a metal, such as copper (e.g., Daniell cell), or silver (e.g., Silver-oxide cell).
Terminology
Anode and cathode
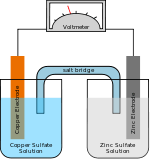
The plate that carries the positive terminal (usually carbon) is termed the anode and the plate that carries the negative terminal (usually zinc) is termed the cathode. This is the reverse of the terminology used in an electrolytic cell. The reason is that the terms are related to the passage of electric current through the electrolyte, not the external circuit
Inside the cell the anode is the electrode where chemical oxidation occurs, as it donates electrons to the circuit. The cathode is defined as the electrode where chemical reduction occurs, as it accepts electrons from the circuit.
Outside the cell, different terminology is used. Since the anode accepts electrons from the electrolyte, it becomes negatively charged and is therefore connected to the terminal marked "−" on the outside of the cell. The cathode, meanwhile, donates electrons to the electrolyte, so it becomes positively charged and is therefore connected to the terminal marked "+" on the outside of the cell.[1]
Old textbooks sometimes contain different terminology that can cause confusion to modern readers. For example, a 1911 textbook by Ayrton and Mather[2] describes the electrodes as the "positive plate" and "negative plate" in a way that contradicts modern usage.
See also
| Wikimedia Commons has media related to Electric batteries. |
References
- ↑ John S. Newman, Karen E. Thomas-Alyea, Electrochemical systems, Wiley-IEEE, 3rd ed. 2004, ISBN 0-471-47756-7
- ↑ W. E. Ayrton and T. Mather, Practical Electricity, Cassell and Company, London, 1911, page 170