Preladenant
From Wikipedia, the free encyclopedia
 | |
|---|---|
| Systematic (IUPAC) name | |
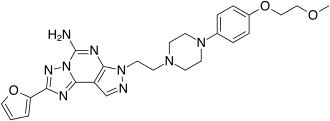
| 2-(2-Furanyl)-7-[2-[4-[4-(2-methoxyethoxy)phenyl]-1-piperazinyl]ethyl]7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine-5-amine | |
| Clinical data | |
| Legal status | Investigational |
| Routes | Oral |
| Identifiers | |
| CAS number | 377727-87-2 |
| ATC code | None |
| PubChem | CID 10117987 |
| UNII | 950O97NUPO |
| Chemical data | |
| Formula | C25H29N9O3 |
| Mol. mass | 503.555 g/mol |
| SMILES
| |
| |
| | |
Preladenant (SCH 420814) was a drug that was developed by Schering-Plough which acted as a potent and selective antagonist at the adenosine A2A receptor. It was being researched as a potential treatment for Parkinson's disease.[1] Positive results were reported in Phase II clinical trials in humans,[2] but it did not prove itself to be more effective than a placebo during Phase III trials, and so was discontinued in May 2013.[3]
References
- ↑ Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Reggiani A, Hunter JC (March 2009). "Characterization of the Potent and Highly Selective A2A Receptor Antagonists Preladenant and SCH 412348 in Rodent Models of Movement Disorders and Depression". The Journal of Pharmacology and Experimental Therapeutics 330 (1): 294–303. doi:10.1124/jpet.108.149617. PMID 19332567.
- ↑ Hauser, R. A.; Cantillon, M.; Pourcher, E.; Micheli, F.; Mok, V.; Onofrj, M.; Huyck, S.; Wolski, K. (2011). "Preladenant in patients with Parkinson's disease and motor fluctuations: a phase 2, double-blind, randomised trial". The Lancet Neurology 10 (3): 221–229. doi:10.1016/S1474-4422(11)70012-6.
- ↑ http://bigstory.ap.org/article/merck-ends-development-parkinsons-disease-drug
| ||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.