Polychlorinated terphenyl
From Wikipedia, the free encyclopedia
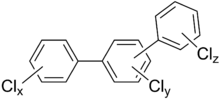
General chemical structure of polychlorinated triphenyls where 0≤x≤5 and 0≤y≤4 and 0≤z≤5
Polychlorinated terphenyls (PCTs) are a group of chlorine derivatives of terphenyls. They are chemically related to polychlorinated biphenyls and have similar chemical properties. They have very low electrical conductivity, high heat stability, and high resistance to alkalies and strong acids.[1] They are non-flammable and insoluble in water.
Typically produced and used as mixtures with varying degrees of chlorination, PCTs were once used as heat transfer agents in electric transformers, as plasticizers, as lubricating oils, and as flame-retardants.[1][2] Their production and use has been largely phased out due to environmental and safety concerns.[2]
International trade in PCTs is regulated by the Rotterdam Convention.
References
- ↑ 1.0 1.1 Filyk, G. (2011-07-01). "Polychlorinated terphenyls" (pdf). United Nations Economic Commission for Europe.
- ↑ 2.0 2.1 U. S. Environmental Protection Agency (1984-03-26). "Chlorinated Terphenyl: Submission of Notice of Manufacture or Importation" (pdf). Federal Register 49 (59): 11181. 40 CFR Part 704 Final rule.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.