Polycaprolactone
| Polycaprolactone | ||
|---|---|---|
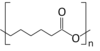 | ||
| IUPAC name (1,7)-polyoxepan-2-one | ||
| Other names 2-Oxepanone homopolymer | ||
| Identifiers | ||
| Abbreviations | PCL | |
| CAS number | 24980-41-4 | |
| Properties | ||
| Molecular formula | (C6H10O2)n | |
| Density | 1.145 g/cm3 | |
| Melting point | 60 °C (140 °F) | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60°C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the manufacture of speciality polyurethanes. Polycaprolactones impart good water, oil, solvent and chlorine resistance to the polyurethane produced.
This polymer is often used as an additive for resins to improve their processing characteristics and their end use properties (e.g., impact resistance). Being compatible with a range of other materials, PCL can be mixed with starch to lower its cost and increase biodegradability or it can be added as a polymeric plasticizer to PVC.
Polycaprolactone is also used for splinting, modeling, and as a feedstock for prototyping systems such as a 3D Printer, where it is used for Fused Filament Fabrication (similar to the Stratasys' Fused Deposition Modeling technique).
Synthesis
PCL is prepared by ring opening polymerization of ε-caprolactone using a catalyst such as stannous octoate. Recently a wide range of catalysts for the ring opening polymerization of caprolactone have been reviewed.[1]

Biomedical applications
PCL is degraded by hydrolysis of its ester linkages in physiological conditions (such as in the human body) and has therefore received a great deal of attention for use as an implantable biomaterial. In particular it is especially interesting for the preparation of long term implantable devices, owing to its degradation which is even slower than that of polylactide.
PCL has been approved by the Food and Drug Administration (FDA) in specific applications used in the human body as (for example) a drug delivery device, suture (sold under the brand name Monocryl or generically), or adhesion barrier.[citation needed] It is being investigated as a scaffold for tissue repair via tissue engineering, GBR membrane. It has been used as the hydrophobic block of amphiphilic synthetic block copolymers used to form the vesicle membrane of polymersomes.
It is also used in housing applications.
A variety of drugs have been encapsulated within PCL beads for controlled release and targeted drug delivery.[2]
The major impurities in the medical grade are toluene (<890 ppm, usually about 100 ppm) and tin (<200 ppm).[citation needed]
In dentistry (as composite named Resilon), it is used as a component of "night guards" (dental splints) and in root canal filling. It performs like gutta-percha, has the similar handling properties, and for retreatment purposes may be softened with heat, or dissolved with solvents like chloroform. Similar to gutta-percha, there are master cones in all ISO sizes and accessory cones in different sizes and taper available. The major difference between the polycaprolactone-based root canal filling material (Resilon and Real Seal) and gutta-percha is that the PCL-based material is biodegradable[3] but gutta-percha is not. There is lack of consensus in the expert dental community as to whether a biodegradable root canal filling material, such as Resilon or Real Seal is desirable.
Hobbyist and prototyping

PCL also has many applications in the hobbyist market (sold under various tradenames, such as "InstaMorph", "Friendly Plastic", "ShapeLock", "PolyMorph", "Plastimake", "Protoplast", "Plaast" etc.). It has physical properties of a very tough, nylon-like plastic that melts to a putty-like consistency at only 60 °C, easily achieved by immersing in hot water. PCL's specific heat and conductivity are low enough that it is not hard to handle at this temperature. This makes it ideal for small-scale modeling, part fabrication, repair of plastic objects, and rapid prototyping where heat resistance is not needed. Though molten PCL readily sticks to many other plastics, if the surface is cooled, the stickiness can be minimized while still leaving the mass pliable.
Biodegradation
Firmicutes and proteobacteria can degrade PCL. [4] Penicillium sp. strain 26-1 can degrade high density PCL; though not as quickly as thermotolerant Aspergillus sp. strain ST-01. Species of clostridium can degrade PCL under anaerobic conditions.
See also
- Silicone
- Fimo
- Silly Putty
- Sellotape
- Duct Tape
- Sugru
References
- ↑ Labet, Marianne; Thielemans, Wim (2009). "Synthesis of polycaprolactone: a review". Chemical Society Reviews 38 (12): 3484–3504. doi:10.1039/B820162P. PMID 20449064.
- ↑ Bhavsar, MD; Amiji, MM. "Development of Novel Biodegradable Polymeric Nanoparticles-in-Microsphere Formulation for Local Plasmid DNA Delivery in the Gastrointestinal Tract". AAPS PharmSciTech 9 (1): 288–294. doi:10.1208/s12249-007-9021-9. PMC 2976886.
- ↑ Hiraishi, Noriko, et al. "Susceptibility of a Polycaprolactone-based Root Canal–filling Material to Degradation. III. Turbidimetric Evaluation of Enzymatic Hydrolysis." Journal of endodontics 33.8 (2007): 952-956.
- ↑ Yutaka Tokiwa; Buenaventurada P. Calabia; Charles U. Ugwu; Seiichi Aiba (September 2009). "Biodegradability of Plastics". International Journal of Molecular Science 9: 3722–3742.
External links
- Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A (June 2004). "Poly-epsilon-caprolactone microspheres and nanospheres: an overview". Int J Pharm 278 (1): 1–23. doi:10.1016/j.ijpharm.2004.01.044. PMID 15158945.