Polyamide
A polyamide is a macromolecule with repeating units linked by amide bonds. [1] They can occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids, and sodium poly(aspartate). Synthetic polyamides are commonly used in textiles, automotives, carpet and sportswear due to their extreme durability and strength. Transportation is the major consumer, accounting for 35% of polyamide (PA) consumption. [2]
Classification
According to the composition of their main chain, polyamides are classified as follows:
| Polyamide family | Main chain | Examples of polyamides | Examples of commercial products |
|---|---|---|---|
| Aliphatic polyamides | Aliphatic | PA 6 and PA 66 | Nylon from DuPont, Technyl from Rhodia, Rilsan and Rilsamid from Arkema |
| Polyphthalamides | Semi-aromatic | PA 6T = hexamethylenediamine + terephthalic acid | Trogamid from Evonik Industries, Amodel from Solvay |
| Aramides = aromatic polyamides | Aromatic | Paraphenylenediamine + terephthalic acid | Kevlar and Nomex from DuPont, Teijinconex, Twaron and Technora from Teijin, Kermel from Kermel, and Spectra from Honeywell. |
According to the number of repeating units' types, polyamides can be:
- homopolymers :
- PA 6 : [NH−(CH2)5−CO]n made from ε-Caprolactam ;
- PA 66 : [NH−(CH2)6−NH−CO−(CH2)4−CO]n made from hexamethylenediamine and adipic acid;
- copolymers :
- PA 6/66 : [NH-(CH2)6−NH−CO−(CH2)4−CO]n−[NH−(CH2)5−CO]m made from caprolactam, hexamethylenediamine and adipic acid ;
- PA 66/610 : [NH−(CH2)6−NH−CO−(CH2)4−CO]n−[NH−(CH2)6−NH−CO−(CH2)8−CO]m made from hexamethylenediamine, adipic acid and sebacic acid.
According to their crystallinity, polyamides can be:
- semi-crystalline:
- high crystallinity : PA46 et PA 66 ;
- low crystallinity : PA mXD6 made from m-xylylenediamine and adipic acid;
- amorphous : PA 6I made from hexamethylenediamine and isophthalic acid.
According to this classification, PA66, for example, is an aliphatic semi-crystalline homopolyamide.
Production from monomers
The amide link is produced from the condensation reaction of an amino group and a carboxylic acid or acid chloride group. A small molecule, usually water, or hydrogen chloride, is eliminated.
The amino group and the carboxylic acid group can be on the same monomer, or the polymer can be constituted of two different bifunctional monomers, one with two amino groups, the other with two carboxylic acid or acid chloride groups.
Amino acids can be taken as examples of single monomer (if the difference between R groups is ignored) reacting with identical molecules to form a polyamide:
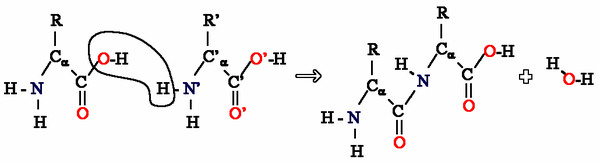
Aramid (pictured below) is made from two different monomers which continuously alternate to form the polymer and is an aromatic polyamide:

See also
References
- ↑ Palmer, R. J. 2001. Polyamides, Plastics. Encyclopedia Of Polymer Science and Technology. doi:10.1002/0471440264.pst251
- ↑ Market Study Engineering Plastics, Ceresana, Sep 2013