Planck charge
In physics, the Planck charge, denoted by 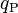 , is one of the base units in the system of natural units called Planck units. It is a quantity of electric charge defined in terms of fundamental physical constants.
, is one of the base units in the system of natural units called Planck units. It is a quantity of electric charge defined in terms of fundamental physical constants.
The Planck charge is defined as:[1] [2]
where:
 is the speed of light in the vacuum,
is the speed of light in the vacuum, is Planck's constant,
is Planck's constant, 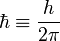 is the reduced Planck constant,
is the reduced Planck constant, 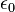 is the permittivity of free space
is the permittivity of free space is the elementary charge
is the elementary charge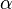 = (137.03599911)−1 is the fine structure constant.
= (137.03599911)−1 is the fine structure constant.
The Planck charge is 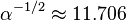 times greater than the elementary charge e carried by an electron.
times greater than the elementary charge e carried by an electron.
The Gaussian cgs units are defined so that  , in which case
, in which case 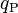 has the following simple form:
has the following simple form:
It is customary in theoretical physics to adopt the Lorentz–Heaviside units (also known as rationalized cgs). When made natural ( ) they are like the SI system with
) they are like the SI system with  . Therefore it is more appropriate to define the Planck charge as
. Therefore it is more appropriate to define the Planck charge as
When charges are measured in units of  , i.e., when
, i.e., when  is set equal to 1, we obtain
is set equal to 1, we obtain 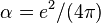 , which is commonly used in theoretical physics. In contrast, in (non-rationalized) natural cgs units where
, which is commonly used in theoretical physics. In contrast, in (non-rationalized) natural cgs units where 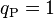 we have
we have  .
.
See also
Notes and references
- ↑ Stock, Michael; Witt, Thomas J (2006). "CPEM 2006 round table discussion 'Proposed changes to the SI'". Metrologia 43 (6): 583. Bibcode:2006Metro..43..583S. doi:10.1088/0026-1394/43/6/014.
- ↑ Pavšič, Matej (2001). The Landscape of Theoretical Physics: A Global View. Dordrecht: Kluwer Academic. pp. 347–352. ISBN 0-7923-7006-6.
| ||||||||


