Pirlimycin
From Wikipedia, the free encyclopedia
 | |
|---|---|
| Systematic (IUPAC) name | |
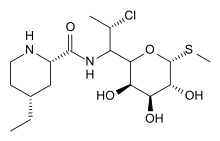
| Methyl(2S-cis)-7-chloro-6,7,8-trideoxy-6[[(4-ethyl-2-piperidinyl) carbonyl]amino]-1-thio-L-threo-α-D-galacto-octopyranoside | |
| Clinical data | |
| Legal status | ? |
| Routes | Intramammary |
| Identifiers | |
| CAS number | 79548-73-5 |
| ATCvet code | QJ51FF90 |
| UNII | LM19JT6G5K |
| KEGG | D08391 |
| ChEMBL | CHEMBL1652611 |
| Chemical data | |
| Formula | C17H31ClN2O5S |
| Mol. mass | 410.164 g/mol |
| SMILES
| |
| | |
Pirlimycin hydrochloride belongs to the lincosamide class of antimicrobials. Under the trade name Pirsue,[1] it is used in the treatment of mastitis in cattle.
Activity
Pirlimycin is active against Gram-positive bacteria, specifically Staphylococcus aureus and coagulase negative species of Staphylococcus and Streptococcus. It has no activity against Gram-negative bacteria.[2]
Mechanism of action
It is bacteriostatic and acts by inhibiting bacterial protein synthesis via binding with the 50S subunit of the ribosome.
References
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.