Pinoresinol
| Pinoresinol | ||
|---|---|---|
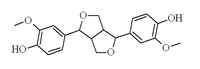 | ||
| IUPAC name 4-[(3S,3aR,6S,6aR)-6-(4-hydroxy-3-methoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2-methoxyphenol | ||
| Other names (+)-Pinoresinol | ||
| Identifiers | ||
| CAS number | 487-36-5 | |
| PubChem | 73399 | |
| ChEMBL | CHEMBL487611 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C20H22O6 | |
| Molar mass | 358.38 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Pinoresinol is a lignan found in Styrax sp.[1] and in Forsythia suspensa.[2] It is also found in the caterpillar of the cabbage butterfly, Pieris rapae where it serves as a defence against ants.[3]
In food, it is found in sesame seed, in Brassica vegetables[4] and in olive oil.[5]
Biosynthesis
A first dirigent protein was discovered in Forsythia intermedia. This protein has been found to direct the stereoselective biosynthesis of (+)-pinoresinol from coniferyl alcohol monomers.[6] Recently, a second, enantiocomplementary dirigent protein was identified in Arabidopsis thaliana, which directs enantioselective synthesis of (-)-pinoresinol.[7]
/-/(%2B)-Pinoresinol_Biosynthesis.png)

Pharmacology
Pinoresinol inhibits the enzyme α-glucosidase in vitro and may therefore act as a hypoglycemic agent.[8] A study involving extra virgin olive oil showed that pinoresinol possess in vitro chemoprevention properties. Increased apoptosis and cellular arrest at the G2/M stage in p53-proficient cells occurred.[9]
Metabolism into enterolignans
Pinoresinol, along with other plant lignans, are converted into enterolignans by intestinal microflora in the human body.[10]
See also
References
- ↑ Pastrorova et al. (1997)
- ↑ Davin, Laurence B.; Bedgar, Diana L.; Katayama, Takeshi; Lewis, Norman G. (1992). "On the stereoselective synthesis of (+)-pinoresinol in Forsythia suspensa from its achiral precursor, coniferyl alcohol". Phytochemistry 31 (11): 3869–74. doi:10.1016/S0031-9422(00)97544-7. PMID 11536515.
- ↑ Schroeder, F. C.; Del Campo, M. L.; Grant, J. B.; Weibel, D. B.; Smedley, S. R.; Bolton, K. L.; Meinwald, J.; Eisner, T. (2006). "Pinoresinol: A lignol of plant origin serving for defense in a caterpillar". Proceedings of the National Academy of Sciences 103 (42): 15497–501. doi:10.1073/pnas.0605921103. PMC 1622851. PMID 17030818.
- ↑ Milder, Ivon E. J.; Arts, Ilja C. W.; Putte, Betty van de; Venema, Dini P.; Hollman, Peter C. H. (2007). "Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". British Journal of Nutrition 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- ↑ Owen, R.W; Giacosa, A; Hull, W.E; Haubner, R; Spiegelhalder, B; Bartsch, H (2000). "The antioxidant/anticancer potential of phenolic compounds isolated from olive oil". European Journal of Cancer 36 (10): 1235–47. doi:10.1016/S0959-8049(00)00103-9. PMID 10882862.
- ↑ Davin LB, Wang HB, Crowell AL, et al. (1997). "Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center". Science 275 (5298): 362–6. doi:10.1126/science.275.5298.362. PMID 8994027.
- ↑ Pickel B, Constantin M-A, Pfannsteil J, Conrad J, Beifuss U, Schaffer A (March 2007). "An Enantiocomplementary Dirigent Protein for the Enantioselective Laccase-Catalyzed Oxidative Coupling of Phenols". Angewandte Chemistry 53 (4): 273–284. doi:10.1007/s10086-007-0892-x.
- ↑ Wikul, A; Damsud, T; Kataoka, K; Phuwapraisirisan, P (2012). "(+)-Pinoresinol is a putative hypoglycemic agent in defatted sesame (Sesamum indicum) seeds though inhibiting α-glucosidase". Bioorganic & Medicinal Chemistry Letters 22 (16): 5215–7. doi:10.1016/j.bmcl.2012.06.068. PMID 22818971.
- ↑ Fini, L; Hotchkiss, E; Fogliano, V; Graziani, G; Romano, M; De Vol, EB; Qin, H; Selgrad, M et al. (2008). "Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines". Carcinogenesis 29 (1): 139–46. doi:10.1093/carcin/bgm255. PMID 17999988.
- ↑ Milder, IE; Arts, IC; Van De Putte, B; Venema, DP; Hollman, PC (2005). "Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". The British journal of nutrition 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
| |||||||||||||||||