Pinacolone
| Pinacolone | |
|---|---|
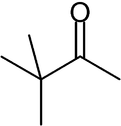 | |
| IUPAC name 3,3-Dimethyl-2-butanone | |
| Other names t-Butyl methyl ketone | |
| Identifiers | |
| CAS number | 75-97-8 |
| PubChem | 6416 |
| ChemSpider | 6176 |
| EC number | 200-920-4 |
| UN number | 1224 |
| MeSH | Pinacolone |
| RTECS number | EL7700000 |
| Beilstein Reference | 1209331 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H12O |
| Molar mass | 100.16 g mol−1 |
| Appearance | Colorless liquid |
| Density | 0.801 g cm-3 |
| Melting point | 10 °C; 50 °F; 283 K |
| Boiling point | 103 to 106 °C; 217 to 223 °F; 376 to 379 K |
| Hazards | |
| MSDS | External MSDS |
| EU classification | |
| R-phrases | R11, R22 |
| S-phrases | S9, S16, S29, S33 |
| NFPA 704 |
 4
1
0
|
| Flash point | 5 °C; 41 °F; 278 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. Pinacolone It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions (hydrogenation, reductive amination, etc.).
Preparation
Most famously, at least in the classroom, pinacolone arises by the pinacol rearrangement, which occurs by protonation of pinacol (2,3-dimethylbutane-2,3-diol).[1]
Industrially pinacolone is made by the hydrolysis of 4,4,5-trimethyl-1,3-dioxane, which is the product of isoprene and formaldehyde via the Prins reaction. It also is generated by ketonization of pivalic acid and acetic acid or acetone over metal oxide catalysts. 3-Methylbutanal is a starting material for 2,3-dimethyl-2-butene, which in turn is converted to pinacolone. Pinacolone can also be produced from 2-methy-2-butanol when reacted with C5 alcohols.[2]
Uses
Pinacolone is produced in large amounts for use in fungicides, herbicides, and pesticides.
References
- ↑ G. A. Hill and E. W. Flosdorf (1941), "Pinacolone", Org. Synth.; Coll. Vol. 1: 462
- ↑ Siegel, H; Eggersdorfer (2012). "Ketones". Ullman's Encyclopedia of Chemistry. 5 20 (6). doi:10.1002/14356007.a15_077.
