Picoline
Picoline refers to three different methylpyridine isomers, all with the chemical formula C6H7N and a molar mass of 93.13 g mol−1. All three are colourless liquids at room temperature and pressure and are miscible with water and most organic solvents. Data for the three compounds are summarized in the table.
| Name(s) | CAS# | m.p. (°C) | b.p. (°C) | pKa of pyridinium ion | structure |
|---|---|---|---|---|---|
| 2-Methylpyridine, α-picoline | [109-06-8] | -66.7 | 129.4 | 5.96 | 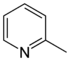 |
| 3-Methylpyridine, β-picoline | [108-99-6] | -18 | 141 | 5.63 | 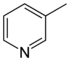 |
| 4-Methylpyridine, γ-picoline | [108-89-4] | 3.6 | 145.4 | 5.98 | 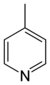 |
The CAS number of an unspecified picoline isomer is [1333-41-1]
Environmental properties
Picolines exhibit greater volatility and are more slowly degraded than their carboxylic acid counterparts. Volatilization is much less extensive in soil than water, owing to sorption of the compounds to soil clays and organic matter.[1] Picoline degradation appears to be mediated primarily by bacteria, with the majority of isolates belonging to the Actinobacteria. 3-Methylpyridine degrades more slowly than the other two isomers, likely due to the impact of resonance in the heterocyclic ring. Like most simple pyridine derivatives, the picolines contain more nitrogen than is needed for growth of microorganisms, and excess nitrogen is generally excreted to the environment as ammonium during the degradation process.[2]