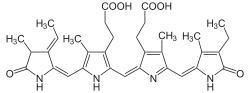
Phycocyanin
| Phycobilisome protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Allophycocyanin 12-mer PDB 1all | |||||||||
| Identifiers | |||||||||
| Symbol | Phycobilisome | ||||||||
| Pfam | PF00502 | ||||||||
| InterPro | IPR001659 | ||||||||
| SCOP | 1cpc | ||||||||
| SUPERFAMILY | 1cpc | ||||||||
| |||||||||
Phycocyanin is a pigment-protein complex from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin.[1] It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble, so they cannot exist within the membrane like carotenoids can. Instead, phycobiliproteins aggregate to form clusters that adhere to the membrane called phycobilisomes. Phycocyanin is a characteristic light blue color, absorbing orange and red light, particularly near 620 nm (depending on which specific type it is), and emits fluorescence at about 650 nm (also depending on which type it is). Allophycocyanin absorbs and emits at longer wavelengths than phycocyanin C or phycocyanin R. Phycocyanins are found in Cyanobacteria (previously called blue-green algae). Phycobiliproteins have fluorescent properties that are used in immunoassay kits. Phycocyanin is from the Greek phyco meaning “algae” and cyanin is from the English word “cyan", which conventionally means a shade of blue-green (close to "aqua") and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue". The product phycocyanin, produced by Aphanizomenon flos-aquae and spirulina, is for example used in the food and beverage industry as the natural coloring agent 'Lina Blue' and is found in sweets and ice cream.
The phycobiliproteins are made of two subunits(alpha and beta) having a protein backbone to which 1-2 linear tetrapyrrole chromophores are covalently bound.
C-phycocyanin is often found in cyanobacteria which thrive around hot springs, as it can be stable up to around 70°C, with identical spectroscopic (light absorbing) behaviours at 20 and 70°C. Thermophiles contain slightly different amino acid sequences making it stable under these higher conditions. Stability of this protein invitro at these temperatures has been shown to be substantially lower. Photo-spectral analysis of the protein after 1 min exposure to 65°C conditions in a purified state demonstrated a 50% loss of tertiary structure.
References
- ↑ Glazer, A.N., 1989. Light guides. Directional energy transfer in a photosynthetic antenna. J. Biol. Chem. 264:1-4
Barsanti, L., & et al. (2008). Oddities and Curiosities in the Algal World. Media, 353-391.
| ||||||||||||||