Phosphite
| Phosphite | ||
|---|---|---|
| Systematic name Phosphite[2] | ||
| Identifiers | ||
| CAS number | 14901-63-4 | |
| PubChem | 107908 | |
| ChemSpider | 97035 | |
| MeSH | Phosphorite | |
| ChEBI | CHEBI:45064 | |
| ChEMBL | CHEMBL1235376 | |
| Gmelin Reference | 68617 | |
| Jmol-3D images | {{#if:[O-]P([O-])[O-]P([O-])([O-])[O-]|Image 1 Image 2 | |
| ||
| ||
| Properties | ||
| Molecular formula | PO33− | |
| Molar mass | 78.9720 g mol−1 | |
| Related compounds | ||
| Other anions | Phosphinite | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
A phosphite in inorganic chemistry is a salt of phosphorous acid, H3PO3 and following the IUPAC naming recommendations the phosphite ion would be PO33−[3] a salt of P(OH)3.
Historically phosphite has referred to salts containing HPO32− and this is because aqueous H3PO3 is not triprotic P(OH)3 but almost exclusively the diprotic HP(O)(OH)2 (IUPAC recommended name of phosphonic acid). The IUPAC recommended name for the HPO32− ion is phosphonate and this naming convention is becoming more common. In the US the IUPAC naming conventions for inorganic compounds are taught at high school.[4] A well known university level text book follows the IUPAC recommendations.[5] In practise any reference to "phosphite" should be investigated carefully to determine which naming convention is being employed.
The term phosphite is also used to mean phosphite ester, an organophosphorus compound with the formula P(OR)3.
Salts containing phosphite, PO33−
Salts containing PO33− cannot be isolated from aqueous solutions of phosphorous acid. Only salts containing H2PO2− or HPO32− are produced. There are reports of a salt Na3PO3 in older literature[6] and the use of sodium metal to remove the third hydrogen in H3PO3 is mentioned in a text book.[5] However if PO33− is produced in aqueous solution or dissolved it would form H2PO2− or HPO32− immediately.[5] Na3PO3 is referred to in many text books on internet sources, often as part of an exercise in naming inorganic compounds, but also as a misprint of the formula for the phosphate salt, Na3PO4.
In contrast to the poor evidence for the existence of PO33− the corresponding arsenic ion, ortho-arsenite, AsO33− is known, and an example is Ag3AsO3 as well as the polymeric meta-arsenite [AsO2−]n.[7] The iso-electronic sulfite ion, SO32− is known from its salts.[7]
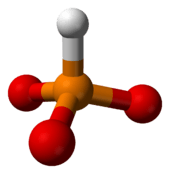
Salts containing HPO32− called phosphonates or phosphites
Many salts containing this ion are known, and many have been investigated structurally, these include (NH4)2HPO3.H2O, CuHPO3.H2O, SnHPO3 and Al2(HPO3)3.4H2O.[8] The structure of the HPO32− is approximately tetrahedral.[1][9]
The isoelectronic bisulfite ion, HSO3− has a similar structure,[7] but this ion tautomerises in solution with HOSO2−.[7]
Salts containing HP(O)2OH− called hydrogenphosphonates or acid phosphites
Acid or hydrogen phosphites (which the IUPAC recommends be called hydrogenphosphonates), containing the ion HP(O)2OH− such as NH4HP(O)2OH, can be prepared.[7] Hydrogen bonding between anions leads to polymeric anionic structures.[9] Recently some others, RbHPHO3, CsHPHO3, TlHPHO3 have been prepared by reacting phosphorous acid with the metal carbonate. These compounds contain a layer polymeric anion consisting of HPO3 tetrahedra linked by hydrogen bonds. These layers are interleaved by layers of metal cations.[10]
Salts containing H2P2O52− called diphosphites or pyrophosphites
Pyrophosphites (diphosphites) can be produced by gently heating acid phosphites under reduced pressure. They contain the ion, H2P2O52− which can be formulated [HP(O)2O−P(O)2H]2−.[7][9]
Synthesis of phosphite esters
Organophosphorus compounds called phosphite esters (or sometimes just phosphites) have the formula (RO)3P. They are prepared by reacting phosphorus trichloride (or phosphorus tribromide) with an alcohol and a tertiary amine.
PCl3 + 3 ROH + 3 R'3N → P(OR)3 + 3R'3NHCl
Use as fungicides
Inorganic phosphites (containing HPO32−) have been applied to crops to combat fungus-like pathogens of the order Oomycetes. The situation is confusing because of the similarity in name between phosphite and phosphate (a major plant nutrient and fertilizer ingredient), and controversial because phosphites have sometimes been advertised as fertilizers, even though they are converted to phosphate too slowly to serve as a plant's main phosphorus source. Lemoynie[11] and others[12] have described this complicated situation and noted that calling phosphites fertilizers avoided the regulatory complication and negative public perceptions that might have been incurred by registering them as fungicides.
See also
- Hypophosphite- H2PO2−
- Organophosphorus
- Phosphine - PH3 and the organic phosphines PR3
- Phosphine oxide - OPR3
- Phosphinite - P(OR)R2
- Phosphonite - P(OR)2R
- Phosphinate - OP(OR)R2
- Phosphonate - organic phosphonates OP(OR)2R
- Phosphate - PO43–
- organophosphate - OP(OR)3
Further reading
- A. Earnshaw, Norman Greenwood (1997). The Chemistry of the Elements - Second Edition. pp. 513–514.
References
- ↑ 1.0 1.1 L. E. Gordon, W. T. A. Harrison (February 2003). "Bis(melaminium) hydrogen phosphite tetrahydrate". Acta Cryst. 59 (2): o195-o197. doi:10.1107/S1600536803001247
- ↑ "Phosphorite - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 ed. N. G. Connelly et al. RSC Publishing http://www.chem.qmul.ac.uk/iupac/bioinorg/
- ↑ Physical setting/ chemistry core curriculum, The University of the State of New York, The State Education Department, http://www.p12.nysed.gov/ciai/mst/pub/chemist.pdf
- ↑ 5.0 5.1 5.2 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- ↑ Modern Inorganic Chemistry, Joseph Mellor, Longmans, Green and Company, 1917
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ Synthesis and crystal structures of aluminum and iron phosphites, D.M. Poojary, Y. Zhang, D.E. Cox, P.R. Rudolf, S. Cheng & A. Clearfield,J. Chem. Crystallogr. 24 (1994) 155-163
- ↑ 9.0 9.1 9.2 Crystal chemistry of inorganic phosphites, J. Loub, Acta Cryst. (1991), B47, 468-473, doi:10.1107/S0108768191002380
- ↑ Kosterina, E. V., Troyanov, S. I., Kemnitz, E. & Aslanov, L. A. (2001). "Synthesis and Crystal Structure of Acid Phosphites RbH2PO3, CsH2PO3, and TlH2PO3". Russian Journal of Coordination Chemistry 27 (7): 458–462. doi:10.1023/A:1011377229855.
- ↑ Phosphites and Phosphates: When Distributors and Growers alike could get confused! by Jean-Pierre Leymonie. Courtesy of New Ag International, September 2007 edition.
- ↑ Thao; Yamakawa (2008). Soil Science and Plant Nutrition 55: 228–234.