Phase boundary
Each phase (i.e. liquid, solid etc.) of physical matter comes to an end at a transitional point, or spatial interface, called a phase boundary, due to the immiscibility of said matter with the matter on the other side of said boundary. This immiscibility is due to at least one difference between the two substances' corresponding physical properties. The behavior of phase boundaries has been a developing subject of interest and an active research field, called interface science, in physics and mathematics for almost two centuries, due partly to phase boundaries naturally arising in many physical processes, such as the capillarity effect, the growth of grain boundaries, the physics of binary alloys, and the formation of snow flakes.
One of the oldest problems in the area dates back to Lame and Clapeyron[1] who studied the freezing of the ground. Their goal was to determine the thickness of solid crust generated by the cooling of a liquid at constant temperature filling the half-space. In 1889, Stefan, while working on the freezing of the ground developed these ideas further and formulated the two phase model which came to be known as the Stefan Problem.[2]
The proof of existence and uniqueness of a solution to the Stefan problem was done in many stages. Proving the general existence of the solutions turned out to be a difficult problem for 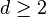 that was finally solved by Enverbek Meirmenov.[3]
that was finally solved by Enverbek Meirmenov.[3]