Periodic table
.svg.png)
The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers, electron configurations (electron shell model), and recurring chemical properties. Elements are presented in order of increasing atomic number (the number of protons in the nucleus). The standard form of the table consists of a grid of elements laid out in 18 columns and 7 rows, with a double row of elements below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that.
The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences.
Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.
All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117 and 118 having yet to be confirmed. The first 98 elements exist naturally although some[n 2] are found only in trace amounts and were initially discovered by synthesis in laboratories. Elements with atomic numbers from 99 to 118 have only been synthesized, or claimed to be so, in laboratories. Production of elements having higher atomic numbers is being pursued, with the question of how the periodic table may need to be modified to accommodate any such additions being a matter of ongoing debate. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.
Layout
| Periodic table | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||
| Alkali metals | Alkaline earth metals | Pnictogens | Chalcogens | Halogens | Noble gases | |||||||||||||||||||||||||
| Period |
||||||||||||||||||||||||||||||
| 2 | ||||||||||||||||||||||||||||||
| 3 | ||||||||||||||||||||||||||||||
| 4 | ||||||||||||||||||||||||||||||
| 5 | ||||||||||||||||||||||||||||||
| 6 | * | |||||||||||||||||||||||||||||
| 7 | ** | |||||||||||||||||||||||||||||
| * Lanthanides | ||||||||||||||||||||||||||||||
| ** Actinides | ||||||||||||||||||||||||||||||
|
This is an 18-column periodic table layout, which has come to be referred to as the common or standard form, on account of its popularity. It is also sometimes referred to as the long form, in comparison to the short form or Mendeleev-style, which omits groups 3–12 by placing their elements into the main groups. The wide periodic table incorporates the lanthanides and the actinides, rather than separating them from the main body of the table. The extended periodic table adds the 8th and 9th periods, including the superactinides. | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
All versions of the periodic table include only chemical elements, not mixtures, compounds, or subatomic particles.[n 3] Each chemical element has a unique atomic number representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never separated in the periodic table; they are always grouped together under a single element. Elements with no stable isotopes have the atomic masses of their most stable isotopes, where such masses are shown, listed in parentheses.[3]
In the standard periodic table, the elements are listed in order of increasing atomic number (the number of protons in the nucleus of an atom). A new row (period) is started when a new electron shell has its first electron. Columns (groups) are determined by the electron configuration of the atom; elements with the same number of electrons in a particular subshell fall into the same columns (e.g. oxygen and selenium are in the same column because they both have four electrons in the outermost p-subshell). Elements with similar chemical properties generally fall into the same group in the periodic table, although in the f-block, and to some respect in the d-block, the elements in the same period tend to have similar properties, as well. Thus, it is relatively easy to predict the chemical properties of an element if one knows the properties of the elements around it.[4]
As of 2013, the periodic table has 114 confirmed elements, comprising elements 1 (hydrogen) to 112 (copernicium), 114 (flerovium) and 116 (livermorium). Elements 113, 115, 117 and 118 have reportedly been synthesised in laboratories however none of these claims have been officially confirmed by the International Union of Pure and Applied Chemistry (IUPAC). As such these elements are currently known only by their systematic element names, based on their atomic numbers.[5]
A total of 98 elements occur naturally; the remaining 16 elements, from einsteinium to copernicium, and flerovium and livermorium, occur only when synthesised in laboratories. Of the 98 elements that occur naturally, 84 are primordial. The other 14 elements occur only in decay chains of primordial elements.[6] No element heavier than einsteinium (element 99) has ever been observed in macroscopic quantities in its pure form.[7]
Grouping methods
Groups
A group or family is a vertical column in the periodic table. Groups usually have more significant periodic trends than periods and blocks, explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group generally have the same electron configurations in their valence shell.[8] Consequently, elements in the same group tend to have a shared chemistry and exhibit a clear trend in properties with increasing atomic number.[9] However in some parts of the periodic table, such as the d-block and the f-block, horizontal similarities can be as important as, or more pronounced than, vertical similarities.[10][11][12]
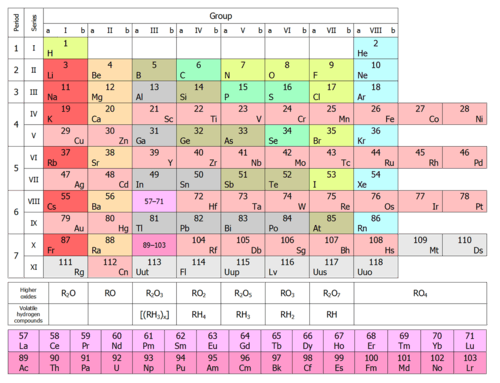
Under an international naming convention, the groups are numbered numerically from 1 to 18 from the leftmost column (the alkali metals) to the rightmost column (the noble gases).[13] Previously, they were known by roman numerals. In America, the roman numerals were followed by either an "A" if the group was in the s- or p-block, or a "B" if the group was in the d-block. The roman numerals used correspond to the last digit of today's naming convention (e.g. the group 4 elements were group IVB, and the group 14 elements was group IVA). In Europe, the lettering was similar, except that "A" was used if the group was before group 10, and "B" was used for groups including and after group 10. In addition, groups 8, 9 and 10 used to be treated as one triple-sized group, known collectively in both notations as group VIII. In 1988, the new IUPAC naming system was put into use, and the old group names were deprecated.[14]
Some of these groups have been given trivial (unsystematic) names, as seen in the table below, although some are rarely used. Groups 3–10 have no trivial names and are referred to simply by their group numbers or by the name of the first member of their group (such as 'the scandium group' for Group 3), since they display fewer similarities and/or vertical trends.[13]
Elements in the same group tend to show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, valence electrons are found farther from the nucleus. From the top, each successive element has a lower ionization energy because it is easier to remove an electron since the atoms are less tightly bound. Similarly, a group has a top to bottom decrease in electronegativity due to an increasing distance between valence electrons and the nucleus.[15] There are exceptions to these trends, however, an example of which occurs in group 11 where electronegativity increases farther down the group.[16]
| Groups in the periodic table | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group number | 1 | 2 | 3b | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| CAS (US) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | |||
| old IUPAC (European) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIII | IB | IIB | IIIB | IVB | VB | VIB | VIIB | Group 0 | |||
| Trivial name | Alkali metals | Alkaline earth metals | Coinage metalsc | Volatile metalsc | Icosagensc | Crystallogensc | Pnictogens | Chalcogens | Halogens | Noble gases | |||||||||
| Name by element | Lithium group | Beryllium group | Scandium group | Titanium group | Vanadium group | Chromium group | Manganese group | Iron group | Cobalt group | Nickel group | Copper group | Zinc group | Boron group | Carbon group | Nitrogen group | Oxygen group | Fluorine group | Helium or Neon group | |
| Period 1 | Ha | He | |||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Period 5 | Rb | Sr | f-blockb | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Period 6 | Cs | Ba | lanthanides | Lub | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period 7 | Fr | Ra | actinides | Lrb | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
| a Hydrogen (H), while placed in column 1, is not considered to be an alkali metal. | |||||||||||||||||||
| b Group 3: depending on the source: Lutetium (Lu) and Lawrencium (Lr) may be included; the f-block (with 14 lanthanides and 14 actinides) may be included. | |||||||||||||||||||
| c This group name is not recommended by IUPAC. | |||||||||||||||||||
| Large version | |||||||||||||||||||
Periods
A period is a horizontal row in the periodic table. Although groups generally have more significant periodic trends, there are regions where horizontal trends are more significant than vertical group trends, such as the f-block, where the lanthanides and actinides form two substantial horizontal series of elements.[17]
Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus.[18] This decrease in atomic radius also causes the ionization energy to increase when moving from left to right across a period. The more tightly bound an element is, the more energy is required to remove an electron. Electronegativity increases in the same manner as ionization energy because of the pull exerted on the electrons by the nucleus.[15] Electron affinity also shows a slight trend across a period. Metals (left side of a period) generally have a lower electron affinity than nonmetals (right side of a period), with the exception of the noble gases.[19]
Blocks
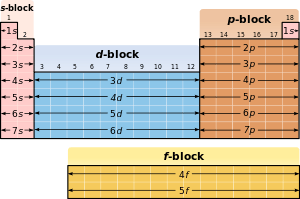
The different regions of the periodic table are sometimes referred to as blocks in recognition of the sequence in which the electron shells of the elements are filled. Each block is named according to the subshell in which the "last" electron notionally resides.[20][n 4] The s-block comprises the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p-block comprises the last six groups which are groups 13 to 18 in IUPAC (3A to 8A in American) and contains, among other elements, all of the metalloids. The d-block comprises groups 3 to 12 in IUPAC (or 3B to 2B in American group numbering) and contains all of the transition metals. The f-block, usually offset below the rest of the periodic table, comprises the lanthanides and actinides.[21]
Categories
The elements can be conveniently classified according to their shared physical and chemical properties into the major categories of metals, metalloids and nonmetals. Metals are generally located to the left and bottom of the periodic table. They are ordinarily shiny, highly conducting solids which form alloys with one another and salt-like ionic compounds with nonmetals. Nonmetals are located to the right and top. They are mostly coloured or colourless insulating gases that form covalent compounds with one another. In between metals and nonmetals are metalloids, which have intermediate or mixed properties.[22]
Metal and nonmetals can be further classified into similar subcategories that show a left to right gradation in metallic to non-metallic properties. The metals are divided into the highly reactive alkali metals, through the less reactive alkaline earth metals, lanthanides and actinides, via the archetypal transition metals, and ending in the physically and chemically weak poor metals. The nonmetals are simply subdivided into the polyatomic nonmetals which, being nearest the metalloids, show some incipient metallic character; the diatomic nonmetals, which are essentially nonmetallic; and the monatomic noble gases, which are almost completely inert and nonmetallic. Specialized groupings such as the refractory metals and the noble metals, which are subsets (in this example) of the transition metals, are also known[23] and occasionally denoted.[24]
Placing the elements into categories and subcategories based on shared properties is imperfect. There is a spectrum of properties within each category and it is not hard to find overlaps at the boundaries, as is the case with most classification schemes.[25] Beryllium, for example, is classified as an alkaline earth metal although its amphoteric chemistry and tendency to mostly form covalent compounds are both attributes of a chemically weak or poor metal. Radon is classified as a nonmetal and a noble gas yet has some cationic chemistry that is more characteristic of a metal. Other classification schemes are possible such as the division of the elements into mineralogical occurrence categories, or crystalline structures. Categorising the elements in this fashion dates back to at least 1869 when Hinrichs[26] wrote that simple boundary lines could be drawn on the periodic table to show elements having like properties, such as the metals and the nonmetals, or the gaseous elements.
Other conventions and variations
In presentations of the periodic table, the lanthanides and the actinides are customarily shown as two additional rows below the main body of the table,[27] with placeholders or else a selected single element of each series (either lanthanum or lutetium, and either actinium or lawrencium, respectively) shown in a single cell of the main table, between barium and hafnium, and radium and rutherfordium, respectively. This convention is entirely a matter of aesthetics and formatting practicality; a rarely used wide-formatted periodic table inserts the lanthanide and actinide series in their proper places, as parts of the table's sixth and seventh rows (periods). Some periodic tables include a dividing line, or equivalent, between metals and nonmetals.[28]
.svg.png) |
.svg.png) |
| Periodic table with f-block separated (left) and inline (right) | |
Periodic trends
Electron configuration

The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered shell 1, shell 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram to the right. The electron configuration for neon, for example, is 1s2 2s2 2p6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell—two in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.[29][30]
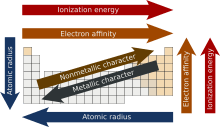
Since the properties of an element are mostly determined by its electron configuration, the properties of the elements likewise show recurring patterns or periodic behaviour, some examples of which are shown in the diagram on the left. It is this periodicity of properties, manifestations of which were noticed well before the underlying theory was developed, that led to the establishment of the periodic law (the properties of the elements recur at varying intervals) and the formulation of the first periodic tables.[29][30]
Atomic radii

Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom; they provided important evidence for the development and confirmation of quantum theory.[31]
The electrons in the 4f-subshell, which is progressively filled from cerium (Z = 58) to lutetium (Z = 71), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanides have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.[32] Hence hafnium has virtually the same atomic radius (and chemistry) as zirconium, and tantalum has an atomic radius similar to niobium, and so forth. This is known as the lanthanide contraction. The effect of the lanthanide contraction is noticeable up to platinum (Z = 78), after which it is masked by a relativistic effect known as the inert pair effect.[33] The d-block contraction, which is a similar effect between the d-block and p-block, is less pronounced than the lanthanide contraction but arises from a similar cause.[32]
Ionization energy

The first ionization energy is the energy it takes to remove one electron from an atom, the second ionization energy is the energy it takes to remove a second electron from the atom, and so on. For a given atom, successive ionization energies increase with the degree of ionization. For magnesium as an example, the first ionization energy is 738 kJ/mol and the second is 1450 kJ/mol. Electrons in the closer orbitals experience greater forces of electrostatic attraction; thus, their removal requires increasingly more energy. Ionization energy becomes greater up and to the right of the periodic table.[33]
Large jumps in the successive molar ionization energies occur when removing an electron from a noble gas (complete electron shell) configuration. For magnesium again, the first two molar ionization energies of magnesium given above correspond to removing the two 3s electrons, and the third ionization energy is a much larger 7730 kJ/mol, for the removal of a 2p electron from the very stable neon-like configuration of Mg2+. Similar jumps occur in the ionization energies of other third-row atoms.[33]
Electronegativity

Electronegativity is the tendency of an atom to attract electrons.[34] An atom's electronegativity is affected by both its atomic number and the distance between the valence electrons and the nucleus. The higher its electronegativity, the more an element attracts electrons. It was first proposed by Linus Pauling in 1932.[35] In general, electronegativity increases on passing from left to right along a period, and decreases on descending a group. Hence, fluorine is the most electronegative of the elements,[n 5] while caesium is the least, at least of those elements for which substantial data is available.[16]
There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon respectively because of the d-block contraction. Elements of the fourth period immediately after the first row of the transition metals have unusually small atomic radii because the 3d-electrons are not effective at shielding the increased nuclear charge, and smaller atomic size correlates with higher electronegativity.[16] The anomalously high electronegativity of lead, particularly when compared to thallium and bismuth, appears to be an artifact of data selection (and data availability)—methods of calculation other than the Pauling method show the normal periodic trends for these elements.[36]
Electron affinity

The electron affinity of an atom is the amount of energy released when an electron is added to a neutral atom to form a negative ion. Although electron affinity varies greatly, some patterns emerge. Generally, nonmetals have more positive electron affinity values than metals. Chlorine most strongly attracts an extra electron. The electron affinities of the noble gases have not been measured conclusively, so they may or may not have slightly negative values.[37]
Electron affinity generally increases across a period. This is caused by the filling of the valence shell of the atom; a group 17 atom releases more energy than a group 1 atom on gaining an electron because it obtains a filled valence shell and is therefore more stable.[37]
A trend of decreasing electron affinity going down groups would be expected. The additional electron will be entering an orbital farther away from the nucleus. As such this electron would be less attracted to the nucleus and would release less energy when added. However, in going down a group, around one-third of elements are anomalous, with heavier elements having higher electron affinities than their next lighter congenors. Largely, this is due to the poor shielding by d and f electrons. A uniform decrease in electron affinity only applies to group 1 atoms.[38]
Metallic character
The lower the values of ionization energy, electronegativity and electron affinity, the more metallic character the element has. Conversely, nonmetallic character increases with higher values of these properties.[39] Given the periodic trends of these three properties, metallic character tends to decrease going across a period and, with some irregularities (mostly) due to poor screening of the nucleus by d and f electrons, and relativistic effects,[40] tends to increase going down a group. Thus, the most metallic elements (such as caesium and francium) are found at the bottom left of traditional periodic tables and the most nonmetallic elements (oxygen, fluorine, chlorine) at the top right. The combination of horizontal and vertical trends in metallic character explains the stair-shaped dividing line between metals and nonmetals found on some periodic tables, and the practice of sometimes categorizing several elements adjacent to that line, or elements adjacent to those elements, as metalloids.[41][42]
History
First systemization attempts

In 1789, Antoine Lavoisier published a list of 33 chemical elements, grouping them into gases, metals, nonmetals, and earths;[43] Chemists spent the following century searching for a more precise classification scheme. In 1829, Johann Wolfgang Döbereiner observed that many of the elements could be grouped into triads based on their chemical properties. Lithium, sodium, and potassium, for example, were grouped together in a triad as soft, reactive metals. Döbereiner also observed that, when arranged by atomic weight, the second member of each triad was roughly the average of the first and the third;[44] this became known as the Law of Triads.[45] German chemist Leopold Gmelin worked with this system, and by 1843 he had identified ten triads, three groups of four, and one group of five. Jean-Baptiste Dumas published work in 1857 describing relationships between various groups of metals. Although various chemists were able to identify relationships between small groups of elements, they had yet to build one scheme that encompassed them all.[44]
In 1858, German chemist August Kekulé observed that carbon often has four other atoms bonded to it. Methane, for example, has one carbon atom and four hydrogen atoms. This concept eventually became known as valency; different elements bond with different numbers of atoms.[46]
In 1862, Alexandre-Emile Béguyer de Chancourtois, a French geologist, published an early form of periodic table, which he called the telluric helix or screw. He was the first person to notice the periodicity of the elements. With the elements arranged in a spiral on a cylinder by order of increasing atomic weight, de Chancourtois showed that elements with similar properties seemed to occur at regular intervals. His chart included some ions and compounds in addition to elements. His paper also used geological rather than chemical terms and did not include a diagram; as a result, it received little attention until the work of Dmitri Mendeleev.[47]
In 1864, Julius Lothar Meyer, a German chemist, published a table with 44 elements arranged by valency. The table showed that elements with similar properties often shared the same valency.[48] Concurrently, William Odling (an English chemist) published an arrangement of 57 elements, ordered on the basis of their atomic weights. With some irregularities and gaps, he noticed what appeared to be a periodicity of atomic weights amongst the elements and that this accorded with 'their usually received groupings.' [49] Odling alluded to the idea of a periodic law but did not pursue it.[50] He subsequently proposed (in 1870) a valence-based classification of the elements.[51]

English chemist John Newlands produced a series of papers from 1863 to 1866 noting that when the elements were listed in order of increasing atomic weight, similar physical and chemical properties recurred at intervals of eight; he likened such periodicity to the octaves of music.[52][53] This so termed Law of Octaves, however, was ridiculed by Newlands' contemporaries, and the Chemical Society refused to publish his work.[54] Newlands was nonetheless able to draft a table of the elements and used it to predict the existence of missing elements, such as germanium.[55] The Chemical Society only acknowledged the significance of his discoveries five years after they credited Mendeleev.[56]
In 1867, Gustavus Hinrichs, a Danish born academic chemist based in America, published a spiral periodic system based on atomic spectra and weights, and chemical similarities. His work was regarded as idiosyncratic, ostentatious and labyrinthine and this may have militated against its recognition and acceptance.[57][58]
Mendeleev's table


Russian chemistry professor Dmitri Mendeleev and German chemist Julius Lothar Meyer independently published their periodic tables in 1869 and 1870, respectively.[59] Mendeleev's table was his first published version; that of Meyer was an expanded version of his (Meyer's) table of 1864.[60] They both constructed their tables by listing the elements in rows or columns in order of atomic weight and starting a new row or column when the characteristics of the elements began to repeat.[61]
The recognition and acceptance afforded to Mendeleev's table came from two decisions he made. The first was to leave gaps in the table when it seemed that the corresponding element had not yet been discovered.[62] Mendeleev was not the first chemist to do so, but he was the first to be recognized as using the trends in his periodic table to predict the properties of those missing elements, such as gallium and germanium.[63] The second decision was to occasionally ignore the order suggested by the atomic weights and switch adjacent elements, such as tellurium and iodine, to better classify them into chemical families. With the development of theories of atomic structure, it became apparent that Mendeleev had unintentionally listed the elements in order of increasing atomic number or nuclear charge.[64]
The significance of atomic numbers to the organization of the periodic table was not appreciated until the existence and properties of protons and neutrons became understood. Mendeleev's periodic tables used atomic weight instead of atomic number to organize the elements, information determinable to fair precision in his time. Atomic weight worked well enough in most cases to (as noted) give a presentation that was able to predict the properties of missing elements more accurately than any other method then known. Substitution of atomic numbers, once understood, gave a definitive, integer-based sequence for the elements, still used today even as new synthetic elements are being produced and studied.[65]
Further development
In 1871, Mendeleev published an updated form of periodic table (shown at left), as well as giving detailed predictions for the elements he had earlier noted were missing, but should exist.[66] These gaps were subsequently filled as chemists discovered additional naturally occurring elements.[67] It is often stated that the last naturally occurring element to be discovered was francium (referred to by Mendeleev as eka-caesium) in 1939.[68] However, plutonium, produced synthetically in 1940, was identified in trace quantities as a naturally occurring primordial element in 1971,[69] and by 2011 it was known that all the elements up to californium can occur naturally as trace amounts in uranium ores by neutron capture and beta decay.[6]
The popular[70] periodic table layout, also known as the common or standard form (as shown at various other points in this article), is attributable to Horace Groves Deming. In 1923, Deming, an American chemist, published short (Mendeleev style) and medium (18-column) form periodic tables.[71][n 6] Merck and Company prepared a handout form of Deming's 18-column medium table, in 1928, which was widely circulated in American schools. By the 1930s Deming's table was appearing in handbooks and encyclopaedias of chemistry. It was also distributed for many years by the Sargent-Welch Scientific Company.[72][73][74]
With the development of modern quantum mechanical theories of electron configurations within atoms, it became apparent that each period (row) in the table corresponded to the filling of a quantum shell of electrons. Larger atoms have more electron sub-shells, so later tables have required progressively longer periods.[75]

In 1945, Glenn Seaborg, an American scientist, made the suggestion that the actinide elements, like the lanthanides were filling an f sub-level. Before this time the actinides were thought to be forming a fourth d-block row. Seaborg's colleagues advised him not to publish such a radical suggestion as it would most likely ruin his career. As Seaborg considered he did not then have a career to bring into disrepute, he published anyway. Seaborg's suggestion was found to be correct and he subsequently went on to win the 1951 Nobel prize in chemistry for his work in synthesizing actinide elements.[76][77][n 7]
Although minute quantities of some transuranic elements occur naturally,[6] they were all first discovered in laboratories. Their production has expanded the periodic table significantly, the first of these being neptunium, synthesized in 1939.[78] Because many of the transuranic elements are highly unstable and decay quickly, they are challenging to detect and characterize when produced. There have been controversies concerning the acceptance of competing discovery claims for some elements, requiring independent review to determine which party has priority, and hence naming rights. The most recently accepted and named elements are flerovium (element 114) and livermorium (element 116), both named on 31 May 2012.[79] In 2010, a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, claimed to have synthesized six atoms of ununseptium (element 117), making it the most recently claimed discovery.[80]
Alternative layouts
.svg.png)
There are many periodic tables with layouts other than that of the common or standard form. Within 100 years of the appearance of Mendeleev's table in 1869 it has been estimated that around 700 different periodic table versions were published.[81] As well as numerous rectangular variations, other periodic table formats have included, for example,[n 8] circular, cubic, cylindrical, edificial (building-like), helical, lemniscate, octagonal prismatic, pyramidal, separated, spherical, spiral, and triangular forms. Such alternatives are often developed to highlight or emphasize chemical or physical properties of the elements that are not as apparent in traditional periodic tables.[81]
A popular[82] alternative layout is that of Theodor Benfey (1960). The elements are arranged in a continuous spiral, with hydrogen at the center and the transition metals, lanthanides, and actinides occupying peninsulas.[83]
Most periodic tables are two-dimensional[6] however three-dimensional tables are known to as far back as at least 1862 (pre-dating Mendeleev's two-dimensional table of 1869). More recent examples include Courtines' Periodic Classification (1925),[84] Wringley's Lamina System (1949),[85] Giguère's Periodic helix (1965)[86][n 9] and Dufour's Periodic Tree (1996).[87] Going one better, Stowe's Physicist's Periodic Table (1989)[88] has been described as being four-dimensional (having three spatial dimensions and one colour dimension).[89]
The various forms of periodic tables can be thought of as lying on a chemistry–physics continuum.[90] Towards the chemistry end of the continuum can be found, as an example, Rayner-Canham's 'unruly'[91] Inorganic Chemist's Periodic Table (2002),[92] which emphasizes trends and patterns, and unusual chemical relationships and properties. Near the physics end of the continuum is Janet's Left-Step Periodic Table (1928). This has a structure which shows a closer connection to the order of electron-shell filling and, by association, quantum mechanics.[93] Somewhere in the middle of the continuum is the ubiquitous common or standard form of periodic table. This is regarded as better expressing empirical trends in physical state, electrical and thermal conductivity, and oxidation numbers, and other properties easily inferred from traditional techniques of the chemical laboratory.[94]
| Janet left-step periodic table | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1s | H | He | ||||||||||||||||||||||||||||||
| 2s | Li | Be | ||||||||||||||||||||||||||||||
| 2p 3s | B | C | N | O | F | Ne | Na | Mg | ||||||||||||||||||||||||
| 3p 4s | Al | Si | P | S | Cl | Ar | K | Ca | ||||||||||||||||||||||||
| 3d 4p 5s | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | ||||||||||||||
| 4d 5p 6s | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | ||||||||||||||
| 4f 5d 6p 7s | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | Fr | Ra |
| 5f 6d 7p 8s | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | Uue | Ubn |
| f-block | d-block | p-block | s-block | |||||||||||||||||||||||||||||
| This form of periodic table is more congruent with the order in which electron shells are filled, as shown in the accompanying sequence in the left margin (read from top to bottom, left to right). The placement of helium (a noble gas) above beryllium (an alkaline earth metal) ordinarily attracts strong criticism from chemists. | ||||||||||||||||||||||||||||||||
| style="line-height:3 pt">| |
Open questions and controversies
Elements with unknown chemical properties
Although all elements up to ununoctium have been discovered, only elements up to hassium (element 108), along with copernicium (element 112), have known chemical properties. The other elements may behave differently from what would be predicted by extrapolation, due to relativistic effects; for example, flerovium has been predicted to possibly exhibit some noble-gas-like properties, even though it is currently placed in the carbon group.[95] More recent experiments have suggested, however, that flerovium behaves chemically like lead, as expected from its periodic table position.[96]
Further periodic table extensions
It is unclear whether new elements will continue the pattern of the current periodic table as period 8, or require further adaptations or adjustments. Seaborg expected the eighth period to follow the previously established pattern exactly, so that it would include a two-element s-block for elements 119 and 120, a new g-block for the next 18 elements, and 30 additional elements continuing the current f-, d-, and p-blocks.[97] More recently, physicists such as Pekka Pyykkö have theorized that these additional elements do not follow the Madelung rule, which predicts how electron shells are filled and thus affects the appearance of the present periodic table.[98]
Element with the highest possible atomic number
The number of possible elements is not known. A very early suggestion made by Elliot Adams in 1911, and based on the arrangement of elements in each horizontal periodic table row, was that elements of atomic weight greater than 256± (which would equate to between elements 99 and 100 in modern-day terms) did not exist.[99] A higher—more recent—estimate is that the periodic table may end soon after the island of stability,[100] which is expected to center around element 126, as the extension of the periodic and nuclides tables is restricted by proton and neutron drip lines.[101] Other predictions of an end to the periodic table include at element 128 by John Emsley,[6] at element 137 by Richard Feynman[102] and at element 155 by Albert Khazan.[6][n 10]
- Bohr model
The Bohr model exhibits difficulty for atoms with atomic number greater than 137, as any element with an atomic number greater than 137 would require 1s electrons to be traveling faster than c, the speed of light.[103] Hence the non-relativistic Bohr model is inaccurate when applied to such an element.
- Relativistic Dirac equation
The relativistic Dirac equation has problems for elements with more than 137 protons. For such elements, the wave function of the Dirac ground state is oscillatory rather than bound, and there is no gap between the positive and negative energy spectra, as in the Klein paradox.[104] More accurate calculations taking into account the effects of the finite size of the nucleus indicate that the binding energy first exceeds the limit for elements with more than 173 protons. For heavier elements, if the innermost orbital (1s) is not filled, the electric field of the nucleus will pull an electron out of the vacuum, resulting in the spontaneous emission of a positron;[105] however, this does not happen if the innermost orbital is filled, so that element 173 is not necessarily the end of the periodic table.[106]
Placement of hydrogen and helium
Hydrogen and helium are often placed in different places than their electron configurations would indicate; hydrogen is usually placed above lithium, in accordance with its electron configuration, but is sometimes placed above fluorine,[107] or even carbon,[107] as it also behaves somewhat similarly to them. Hydrogen is also sometimes placed in its own group, as it does not behave similarly enough to any element to be placed in a group with another.[108] Helium is almost always placed above neon, as they are very similar chemically, although it is occasionally placed above beryllium on account of having a comparable electron shell configuration (helium: 1s2; beryllium: [He] 2s2).[20]
Groups included in the transition metals
The definition of a transition metal, as given by IUPAC, is an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell.[109] By this definition all of the elements in groups 3–11 are transition metals. The IUPAC definition therefore excludes group 12, comprising zinc, cadmium and mercury, from the transition metals category.
Some chemists treat the categories "d-block elements" and "transition metals" interchangeably, thereby including groups 3–12 among the transition metals. In this instance the group 12 elements are treated as a special case of transition metal in which the d electrons are not ordinarily involved in chemical bonding. The recent discovery that mercury can use its d electrons in the formation of mercury(IV) fluoride (HgF4) has prompted some commentators to suggest that mercury can be regarded as a transition metal.[110] Other commentators, such as Jensen,[111] have argued that the formation of a compound like HgF4 can occur only under highly abnormal conditions. As such, mercury could not be regarded as a transition metal by any reasonable interpretation of the ordinary meaning of the term.[111]
Still other chemists further exclude the group 3 elements from the definition of a transition metal. They do so on the basis that the group 3 elements do not form any ions having a partially occupied d shell and do not therefore exhibit any properties characteristic of transition metal chemistry.[112] In this case, only groups 4–11 are regarded as transition metals.
Period 6 and 7 elements in group 3
Although scandium and yttrium are always the first two group 3 elements, the identity of the next two elements is not agreed upon; they are either lanthanum and actinium, or lutetium and lawrencium. Although there are some strong physical and chemical arguments supporting the latter arrangement not all authors are convinced.[113]
Optimal form
The many different forms of periodic table have prompted the question of whether there is an optimal or definitive form of periodic table. The answer to this question is thought to depend on whether the chemical periodicity seen to occur among the elements has an underlying truth, effectively hard-wired into the universe, or if any such periodicity is instead the product of subjective human interpretation, contingent upon the circumstances, beliefs and predilections of human observers. An objective basis for chemical periodicity would settle the questions about the location of hydrogen and helium, and the composition of group 3. Such an underlying truth, if it exists, is thought to have not yet been discovered. In its absence, the many different forms of periodic table can be regarded as variations on the theme of chemical periodicity, each of which explores and emphasizes different aspects, properties, perspectives and relationships of and among the elements.[n 11] The ubiquity of the standard or medium-long periodic table is thought to be a result of this layout having a good balance of features in terms of ease of construction and size, and its depiction of atomic order and periodic trends.[50][114]
See also
|
- Abundance of the chemical elements
- Atomic electron configuration table
- Element collecting
- List of elements
- List of periodic table-related articles
- Table of nuclides
- Timeline of chemical elements discoveries
Notes
- ↑ The noble gases, astatine, francium, and all elements heavier than americium were left out as there is no data for them.
- ↑ The elements discovered initially by synthesis and later in nature are technetium (Z=43), promethium (61), astatine (85), francium (87), neptunium (93), plutonium (94), americium (95), curium (96), berkelium (97) and californium (98).
- ↑ Some tables include an element zero (i.e. a substance composed purely of neutrons), although this is uncommon. See, for example. Philip Stewart's Chemical Galaxy.
- ↑ There is an inconsistency and some irregularities in this convention. Thus, helium is shown in the p-block but is actually an s-block element, and (for example) the d-subshell in the d-block is actually filled by the time group 11 is reached, rather than group 12.
- ↑ While fluorine is the most electronegative of the elements under the Pauling scale, neon is the most electronegative element under other scales, such as the Allen scale.
- ↑ An antecedent of Deming's 18-column table may be seen in Adams' 16-column Periodic Table of 1911. Adams omits the rare earths and the 'radioactive elements' (i.e. the actinides) from the main body of his table and instead shows them as being 'careted in only to save space' (rare earths between Ba and eka-Yt; radioactive elements between eka-Te and eka-I). See: Elliot Q. A. (1911). "A modification of the periodic table". Journal of the American Chemical Society. 33(5): 684–688 (687).
- ↑ A second extra-long periodic table row, to accommodate known and undiscovered elements with an atomic weight greater than bismuth (thorium, protactinium and uranium, for example), had been postulated as far back as 1892. Most investigators, however, considered that these elements were analogues of the third series transition elements, hafnium, tantalum and tungsten. The existence of a second inner transition series, in the form of the actinides, was not accepted until similarities with the electron structures of the lanthanides had been established. See: van Spronsen, J. W. (1969). The periodic system of chemical elements. Amsterdam: Elsevier. p. 315–316, ISBN 0-444-40776-6.
- ↑ See The Internet database of periodic tables for depictions of these kinds of variants.
- ↑ The animated depiction of Giguère's periodic table that is widely available on the internet (including from here) is erroneous, as it does not include hydrogen and helium. Giguère included hydrogen, above lithium, and helium, above beryllium. See: Giguère P.A. (1966). "The "new look" for the periodic system". Chemistry in Canada 18 (12): 36–39 (see p. 37).
- ↑ Karol (2002, p. 63) contends that gravitational effects would become significant when atomic numbers become astronomically large, thereby overcoming other super-massive nuclei instability phenomena, and that neutron stars (with atomic numbers on the order of 1021) can arguably be regarded as representing the heaviest known elements in the universe. See: Karol P. J. (2002). "The Mendeleev–Seaborg periodic table: Through Z = 1138 and beyond". Journal of Chemical Education 79 (1): 60–63.
- ↑ Scerri, one of the foremost authorities on the history of the periodic table (Sella 2013), favoured the concept of an optimal form of periodic table but has recently changed his mind and now supports the value of a plurality of periodic tables. See: Sella A. (2013). 'An elementary history lesson'. New Scientist. 2929, August 13th: 51, accessed September 4th 2013; and Scerri, E. (2013). 'Is there an optimal periodic table and other bigger questions in the philosophy of science.'. August 9th, accessed September 4th, 2013.
References
- ↑ Huheey, Keiter & Keiter, p. 42
- ↑ Siekierski, Slawomir; Burgess, John (2002). Concise chemistry of the elements. Chichester: Horwood Publishing. pp. 35‒36. ISBN 1898563713.
- ↑ Greenwood, pp. 24–27
- ↑ Gray, p. 6
- ↑ Koppenol, W. H. (2002). "Naming of New Elements (IUPAC Recommendations 2002)" (PDF). Pure and Applied Chemistry 74 (5): 787–791. doi:10.1351/pac200274050787.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. ISBN 978-0-19-960563-7.
- ↑ Haire, Richard G. (2006). "Fermium, Mendelevium, Nobelium and Lawrencium". In Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- ↑ Scerri 2007, p. 24
- ↑ Messler, R. W. (2010). The essence of materials for engineers. Sudbury, MA: Jones & Bartlett Publishers. p. 32. ISBN 0763778338.
- ↑ Bagnall, K. W. (1967). "Recent advances in actinide and lanthanide chemistry". In Fields, PR; Moeller, T. Advances in chemistry, Lanthanide/Actinide chemistry. Advances in Chemistry 71. American Chemical Society. pp. 1–12. doi:10.1021/ba-1967-0071. ISBN 0-8412-0072-6
- ↑ Day, M. C.; Selbin, J. (1969). Theoretical inorganic chemistry (2nd ed.). New York, MA: Reinhold Book Corporation. p. 103. ISBN 0763778338.
- ↑ Holman, J.; Hill, G. C. (2000). Chemistry in context (5th ed.). Walton-on-Thames: Nelson Thornes. p. 40. ISBN 0174482760.
- ↑ 13.0 13.1 Leigh, G. J. (1990). Nomenclature of Inorganic Chemistry: Recommendations 1990. Blackwell Science. ISBN 0-632-02494-1.
- ↑ Fluck, E. (1988). "New Notations in the Periodic Table". Pure Appl. Chem. (IUPAC) 60 (3): 431–436. doi:10.1351/pac198860030431. Retrieved 24 March 2012.
- ↑ 15.0 15.1 Moore, p. 111
- ↑ 16.0 16.1 16.2 Greenwood, p. 30
- ↑ Stoker, Stephen H. (2007). General, organic, and biological chemistry. New York: Houghton Mifflin. p. 68. ISBN 978-0-618-73063-6. OCLC 52445586.
- ↑ Mascetta, Joseph (2003). Chemistry The Easy Way (4th ed.). New York: Hauppauge. p. 50. ISBN 978-0-7641-1978-1. OCLC 52047235.
- ↑ Kotz, John; Treichel, Paul; Townsend, John (2009). Chemistry and Chemical Reactivity, Volume 2 (7th ed.). Belmont: Thomson Brooks/Cole. p. 324. ISBN 978-0-495-38712-1. OCLC 220756597.
- ↑ 20.0 20.1 Gray, p. 12
- ↑ Jones, Chris (2002). d- and f-block chemistry. New York: J. Wiley & Sons. p. 2. ISBN 978-0-471-22476-1. OCLC 300468713.
- ↑ Silberberg, M. S. (2006). Chemistry: The molecular nature of matter and change (4th ed.). New York: McGraw-Hill. p. 536. ISBN 0071116583.
- ↑ Manson, S. S.; Halford, G. R. (2006). Fatigue and durability of structural materials. Materials Park, Ohio: ASM International. p. 376. ISBN 0871708256.
- ↑ Bullinger, Hans-Jörg (2009). Technology guide: Principles, applications, trends. Berlin: Springer-Verlag. p. 8. ISBN 9783540885450.
- ↑ Jones, B. W. (2010). Pluto: Sentinel of the outer solar system. Cambridge: Cambridge University Press. pp. 169–71. ISBN 9780521194365.
- ↑ Hinrichs, G. D. (1869). "On the classification and the atomic weights of the so-called chemical elements, with particular reference to Stas’s determinations". Proceedings of the American Association for the Advancement of Science 18 (5): 112–124.
- ↑ Gray, p. 11
- ↑ Jespersen, N. D. (2010). Barron's AP chemistry (5 revised ed.). Hauppauge, NY: Barron's Educational Series. p. 117. ISBN 0764140507.
- ↑ 29.0 29.1 Myers, R. (2003). The basics of chemistry. Westport, CT: Greenwood Publishing Group. pp. 61–67. ISBN 0313316643.
- ↑ 30.0 30.1 Chang, Raymond (2002). Chemistry (7 ed.). New York: McGraw-Hill. pp. 289–310; 340–42. ISBN 0-07-112072-6.
- ↑ Greenwood, p. 27
- ↑ 32.0 32.1 Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd ed.). McGraw-Hill. p. 22. ISBN 978-0-07-112651-9.
- ↑ 33.0 33.1 33.2 Greenwood, p. 28
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Electronegativity".
- ↑ Pauling, L. (1932). "The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms". Journal of the American Chemical Society 54 (9): 3570–3582. doi:10.1021/ja01348a011.
- ↑ Allred, A. L. (1960). "Electronegativity values from thermochemical data". Journal of Inorganic and Nuclear Chemistry (Northwestern University) 17 (3–4): 215–221. doi:10.1016/0022-1902(61)80142-5.
- ↑ 37.0 37.1 Chang, pp. 307–309
- ↑ Huheey, Keiter & Keiter, pp. 42, 880–81
- ↑ Yoder, C. H.; Suydam, F. H.; Snavely, F. A. (1975). Chemistry (2nd ed.). Harcourt Brace Jovanovich. p. 58. ISBN 0-15-506465-7.
- ↑ Huheey, Keiter & Keiter, pp. 880–85
- ↑ Sacks, O (2009). Uncle Tungsten: Memories of a chemical boyhood. New York: Alfred A. Knopf. pp. 191, 194. ISBN 0-375-70404-3.
- ↑ Gray, p. 9
- ↑ Siegfried, Robert (2002). From elements to atoms: a history of chemical composition. Philadelphia, Pennsylvania: Library of Congress Cataloging-in-Publication Data. p. 92. ISBN 0-87169-924-9.
- ↑ 44.0 44.1 Ball, p. 100
- ↑ Horvitz, Leslie (2002). Eureka!: Scientific Breakthroughs That Changed The World. New York: John Wiley. p. 43. ISBN 978-0-471-23341-1. OCLC 50766822.
- ↑ van Spronsen, J. W. (1969). The periodic system of chemical elements. Amsterdam: Elsevier. p. 19. ISBN 0444407766.
- ↑ Annales des Mines history page.
- ↑ Venable, pp. 85–86; 97
- ↑ Odling, W. (2002). "On the proportional numbers of the elements". Quarterly Journal of Science 1: 642–648 (643).
- ↑ 50.0 50.1 Scerri, Eric R. (2011). The periodic table: A very short introduction. Oxford: Oxford University Press. ISBN 978-0199582495.
- ↑ Kaji, M. (2004). "Discovery of the periodic law: Mendeleev and other researchers on element classification in the 1860s". In Rouvray, D. H.; King, R. Bruce. The periodic table: Into the 21st Century. Research Studies Press. pp. 91–122 (95). ISBN 0-86380-292-3.
- ↑ Newlands, John A. R. (20 August 1864). "On Relations Among the Equivalents". Chemical News 10: 94–95.
- ↑ Newlands, John A. R. (18 August 1865). "On the Law of Octaves". Chemical News 12: 83.
- ↑ Bryson, Bill (2004). A Short History of Nearly Everything. Black Swan. pp. 141–142. ISBN 978-0-552-15174-0.
- ↑ Scerri 2007, p. 306
- ↑ Brock, W. H.; Knight, D. M. (1965). "The Atomic Debates: 'Memorable and Interesting Evenings in the Life of the Chemical Society'". Isis (The University of Chicago Press) 56 (1): 5–25. doi:10.1086/349922.
- ↑ Scerri 2007, pp. 87, 92
- ↑ Kauffman, George B. (March 1969). "American forerunners of the periodic law". Journal of Chemical Education 46 (3): 128–135 (132). Bibcode:1969JChEd..46..128K. doi:10.1021/ed046p128.
- ↑ Mendelejew, Dimitri (1869). "Über die Beziehungen der Eigenschaften zu den Atomgewichten der Elemente". Zeitschrift für Chemie (in German): 405–406.
- ↑ Venable, pp. 96–97; 100–102
- ↑ Ball, pp. 100–102
- ↑ Pullman, Bernard (1998). The Atom in the History of Human Thought. Translated by Axel Reisinger. Oxford University Press. p. 227. ISBN 0-19-515040-6.
- ↑ Ball, p. 105
- ↑ Atkins, P. W. (1995). The Periodic Kingdom. HarperCollins Publishers, Inc. p. 87. ISBN 0-465-07265-8.
- ↑ Samanta, C.; Chowdhury, P. Roy; Basu, D.N. (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001.
- ↑ Scerri 2007, p. 112
- ↑ Kaji, Masanori (2002). "D.I. Mendeleev's Concept of Chemical Elements and the Principle of Chemistry". Bull. Hist. Chem. (Tokyo Institute of Technology) 27 (1): 4–16. Retrieved 11 June 2012.
- ↑ Adloff, Jean-Pierre; Kaufman, George B. (25 September 2005). "Francium (Atomic Number 87), the Last Discovered Natural Element". The Chemical Educator. Retrieved 26 March 2007.
- ↑ Hoffman, D. C.; Lawrence, F. O.; Mewherter, J. L.; Rourke, F. M. (1971). "Detection of Plutonium-244 in Nature". Nature 234 (5325): 132–134. Bibcode:1971Natur.234..132H. doi:10.1038/234132a0.
- ↑ Gray, p. 12
- ↑ Deming, Horace G (1923). General chemistry: An elementary survey. New York: J. Wiley & Sons. pp. 160, 165.
- ↑ Abraham, M; Coshow, D; Fix, W. Periodicity:A source book module, version 1.0. New York: Chemsource, Inc. p. 3.
- ↑ Emsley, J (7 March 1985). "Mendeleyev's dream table". New Scientist: 32–36(36).
- ↑ Fluck, E (1988). "New notations in the period table". Pure & Applied Chemistry 60 (3): 431–436 (432). doi:10.1351/pac198860030431.
- ↑ Ball, p. 111
- ↑ Scerri 2007, pp. 270‒71
- ↑ Masterton, William L.; Hurley, Cecile N.; Neth, Edward J. Chemistry: Principles and reactions (7th ed.). Belmont, CA: Brooks/Cole Cengage Learning. p. 173. ISBN 1111427100.
- ↑ Ball, p. 123
- ↑ Barber, Robert C.; Karol, Paul J; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich W. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)". Pure Appl. Chem. 83 (7): 1485. doi:10.1351/PAC-REP-10-05-01.
- ↑ (Russian) "Эксперимент по синтезу 117-го элемента получает продолжение" [Experiment on sythesis of the 117th element is to be continued]. JINR. 2012.
- ↑ 81.0 81.1 Scerri 2007, p. 20
- ↑ Emsely, J; Sharp, R (21 June 2010). "The periodic table: Top of the charts". The Independent.
- ↑ Seaborg, Glenn (1964). "Plutonium: The Ornery Element". Chemistry 37 (6): 14.
- ↑ Mark R. Leach. "1925 Courtines' Periodic Classification". Retrieved 16 October 2012.
- ↑ Mark R. Leach. "1949 Wringley's Lamina System". Retrieved 16 October 2012.
- ↑ Mazurs, E.G. (1974). Graphical Representations of the Periodic System During One Hundred Years. Alabama: University of Alabama Press. p. 111. ISBN 978-0-8173-3200-6.
- ↑ Mark R. Leach. "1996 Dufour's Periodic Tree". Retrieved 16 October 2012.
- ↑ Mark R. Leach. "1989 Physicist's Periodic Table by Timothy Stowe". Retrieved 16 October 2012.
- ↑ Bradley, David (20 July 2011). "At last, a definitive periodic table?". ChemViews Magazine. doi:10.1002/chemv.201000107.
- ↑ Scerri 2007, pp. 285‒86
- ↑ Scerri 2007, p. 285
- ↑ Mark R. Leach. "2002 Inorganic Chemist's Periodic Table". Retrieved 16 October 2012.
- ↑ Scerri, Eric (2008). "The role of triads in the evolution of the periodic table: Past and present". Journal of Chemical Education 85 (4): 585–89 (see p.589). Bibcode:2008JChEd..85..585S. doi:10.1021/ed085p585.
- ↑ Bent, H. A.; Weinhold, F (2007). "Supporting information: News from the periodic table: An introduction to "Periodicity symbols, tables, and models for higher-order valency and donor–acceptor kinships"". Journal of Chemical Education 84 (7): 3–4. doi:10.1021/ed084p1145.
- ↑ Schändel, Matthias (2003). The Chemistry of Superheavy Elements. Dordrecht: Kluwer Academic Publishers. p. 277. ISBN 1-4020-1250-0.
- ↑ Scerri 2011, pp. 142–143
- ↑ Frazier, K. (1978). "Superheavy Elements". Science News 113 (15): 236–238. doi:10.2307/3963006. JSTOR 3963006.
- ↑ Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics 13 (1): 161–168. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- ↑ Elliot, Q. A. (1911). "A modification of the periodic table". Journal of the American Chemical Society 33 (5): 684–688 (688). doi:10.1021/ja02218a004.
- ↑ Glenn Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ↑ Cwiok, S.; Heenen, P.-H.; Nazarewicz, W. (2005). "Shape coexistence and triaxiality in the superheavy nuclei". Nature 433 (7027): 705–9. Bibcode:2005Natur.433..705C. doi:10.1038/nature03336. PMID 15716943.
- ↑ Elert, G. "Atomic Models". The Physics Hypertextbook. Retrieved 2009-10-09.
- ↑ Eisberg, R.; Resnick, R. (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles. Wiley.
- ↑ Bjorken, J. D.; Drell, S. D. (1964). Relativistic Quantum Mechanics. McGraw-Hill.
- ↑ Greiner, W.; Schramm, S. (2008). American Journal of Physics 76. p. 509., and references therein.
- ↑ Ball, Philip (November 2010). "Would Element 137 Really Spell the End of the Periodic Table? Philip Ball Examines the Evidence". Royal Society of Chemistry. Retrieved 2012-09-30.
- ↑ 107.0 107.1 Cronyn, Marshall W. (August 2003). "The Proper Place for Hydrogen in the Periodic Table". Journal of Chemical Education 80 (8): 947–951. Bibcode:2003JChEd..80..947C. doi:10.1021/ed080p947.
- ↑ Gray, p. 14
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "transition element".
- ↑ Xuefang Wang; Lester Andrews; Sebastian Riedel; and Martin Kaupp (2007). "Mercury Is a Transition Metal: The First Experimental Evidence for HgF4". Angew. Chem. Int. Ed. 46 (44): 8371–8375. doi:10.1002/anie.200703710. PMID 17899620.
- ↑ 111.0 111.1 William B. Jensen (2008). "Is Mercury Now a Transition Element?". J. Chem. Educ. 85 (9): 1182–1183. Bibcode:2008JChEd..85.1182J. doi:10.1021/ed085p1182.
- ↑ Rayner-Canham, G; Overton, T. Descriptive inorganic chemistry (4th ed.). New York: W H Freeman. pp. 484–485. ISBN 0716789639.
- ↑ Scerri, E (2012). "Mendeleev's Periodic Table Is Finally Completed and What To Do about Group 3?". Chemistry International 34 (4).
- ↑ Francl, Michelle (May 2009). "Table manners". Nature Chemistry 1 (2): 97–98. Bibcode:2009NatCh...1...97F. doi:10.1038/nchem.183. PMID 21378810.
Bibliography
- Ball, Philip (2002). The Ingredients: A Guided Tour of the Elements. Oxford: Oxford University Press. ISBN 0-19-284100-9.
- Chang, Raymond (2002). Chemistry (7th ed.). New York: McGraw-Hill Higher Education. ISBN 0−07−112072−6 Check
|isbn=value (help). - Gray, Theodore (2009). The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal Publishers. ISBN 978-1-57912-814-2.
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 0-08-022057-6.
- Huheey, JE; Keiter, EA; Keiter, RL. Principles of structure and reactivity (4th ed.). New York: Harper Collins College Publishers. ISBN 0-06-042995-X.
- Moore, John (2003). Chemistry For Dummies. New York: Wiley Publications. p. 111. ISBN 978-0-7645-5430-8. OCLC 51168057.
- Scerri, Eric (2007). The periodic table: Its story and its significance. Oxford: Oxford University Press. ISBN 0-19-530573-6.
- Scerri, Eric R. (2011). The periodic table: A very short introduction. Oxford: Oxford University Press. ISBN 978-0199582495.
- Venable, F P (1896). The development of the periodic law. Easton PA: Chemical Publishing Company.
External links
| Find more about Periodic table at Wikipedia's sister projects | |
| |
Definitions and translations from Wiktionary |
| |
Media from Commons |
| |
Quotations from Wikiquote |
| |
Source texts from Wikisource |
| |
Textbooks from Wikibooks |
| |
Learning resources from Wikiversity |
- 999GCSEHelp. "GCSE Physics - Periodic Table - Information". Retrieved 20 December 2013.
- M. Dayah. "Dynamic Periodic Table". Retrieved 14 May 2012.
- Brady Haran. "The Periodic Table of Videos". University of Nottingham. Retrieved 14 May 2012.
- Mark Winter. "WebElements: the periodic table on the web". University of Sheffield. Retrieved 14 May 2012.
- Mark R. Leach. "The INTERNET Database of Periodic Tables". Retrieved 14 May 2012.
- "Periodic Table of the Elements in Four Hundred Languages". Retrieved 14 May 2012.
| Periodic table | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
|
| |||||||||||||||||||||||||||||||||
| Large version | |||||||||||||||||||||||||||||||||
| |||||||||||||||||