Perfluorohexane
| Perfluorohexane | |
|---|---|
 | |
 | |
| IUPAC name 1,1,1,2,2,3,3,4,4,5,5,6,6,6-tetradecafluorohexane | |
| Other names FC-72, | |
| Identifiers | |
| CAS number | 355-42-0 |
| PubChem | 9639 |
| ChemSpider | 9262 |
| UNII | FX3WJ41CMX |
| KEGG | D05437 |
| ChEBI | CHEBI:39427 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
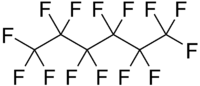
| Molecular formula | C6F14 |
| Molar mass | 338.041845 |
| Melting point | −90 °C; −130 °F; 183 K |
| Boiling point | 56 °C; 133 °F; 329 K |
| Vapor pressure | 27kPa @ 25deg C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Perfluorohexane (C6F14) or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low temperature applications due to its low boiling point of 56°C and freezing point of -90 °C. It is odorless and colorless. Unlike typical hydrocarbons, the structure features a helical carbon backbone.[1]
Oxygen solubility
Because it is biologically inert and chemically stable, perfluorohexane has attracted attention in medicine. Like other fluorocarbons, perfluorohexane dissolves gases including oxygen from the air to a higher concentration than ordinary organic solvents. This effect is attributed to the weak intermolecular forces between perfluorohexane molecules, which allows "space" for gas molecules to partition into the liquid. Animals can be submerged in a bath of perfluorohexane without drowning, as there is sufficient oxygen available in the solvent to allow respiration to continue. This effect has led to the experimental use of perfluorohexane in treating burn victims, as their lungs can be filled with either perfluorohexane vapor or in extreme cases liquid perfluorohexane, allowing breathing to continue without the problems normally seen with pulmonary edema that sometimes occur when the inside of the lungs have been burnt e.g. by inhalation of hot smoke.[2][3]
References
- ↑ John A. Gladysz and Markus Jurisch "Structural, Physical, and Chemical Properties of Fluorous Compounds" in István T. Horváth (Ed.) Topics in Current Chemistry 2011 "Fluorous Chemistry" doi:10.1007/128_2011_282
- ↑ de Abreu MG, Quelhas AD, Spieth P, Brauer G, Knels L, Kasper M, Pino AV, Bleyl JU, Hubler M, Bozza F, Salluh J, Kuhlisch E, Giannella-Neto A, Koch T. Comparative effects of vaporized perfluorohexane and partial liquid ventilation in oleic acid-induced lung injury. Anesthesiology. 2006 Feb;104(2):278-89.
- ↑ Bleyl JU, Ragaller M, Tscho U, Regner M, Hubler M, Kanzow M, Vincent O, Albrecht M. Changes in pulmonary function and oxygenation during application of perfluorocarbon vapor in healthy and oleic acid-injured animals. Critical Care Medicine. 2002 Jun;30(6):1340-7.