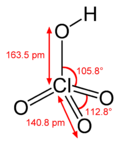
Perchloric acid
| Perchloric acid | |
|---|---|
 |
 |
 | |
| Other names Hyperchloric acid[1] | |
| Identifiers | |
| CAS number | 7601-90-3 |
| PubChem | 24247 |
| ChemSpider | 22669 |
| EC number | 231-512-4 |
| UN number | 1873 |
| ChEBI | CHEBI:29221 |
| ChEMBL | CHEMBL1161634 |
| RTECS number | SC7500000 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | HClO4 |
| Molar mass | 100.46 g/mol |
| Appearance | colorless liquid |
| Density | 1.67 g/cm3 |
| Melting point | -17 C (azeotrope)[2] -112 °C (anhydrous) |
| Boiling point | 203 C (azeotrope)[3] |
| Solubility in water | miscible |
| Acidity (pKa) | ≈ −8[4] |
| Hazards | |
| MSDS | ICSC 1006 |
| EU Index | 017-006-00-4 |
| EU classification | Oxidant (O) Corrosive (C) |
| R-phrases | R5, R8, R35 |
| S-phrases | (S1/2), S23, S26, S36, S45 |
| NFPA 704 |
 0
3
3
OX
|
| Related compounds | |
| Related compounds | Hydrochloric acid Hypochlorous acid Chlorous acid Chloric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Perchloric acid is an inorganic compound with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to approximately 70% are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel. Overall, perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.
Production
Perchloric acid is produced industrially by two routes. The traditional method exploits the high aqueous solubility of sodium perchlorate (209 g/100 mL of water at room temperature). Treatment of such solutions with hydrochloric acid gives perchloric acid, precipitating solid sodium chloride:
- NaClO4 + HCl → NaCl + HClO4
The concentrated acid can be purified by distillation. The alternative route, which is more direct and avoids salts, entails anodic oxidation of aqueous chlorine at a platinum electrode.[5]
Laboratory preparations
Treatment of barium perchlorate with sulfuric acid precipitates barium sulfate, leaving perchloric acid. It also can be made by mixing nitric acid with ammonium perchlorate. The reaction gives nitrous oxide and perchloric acid due to a concurrent reaction involving the ammonium ion.
Properties
Anhydrous perchloric acid is an oily liquid at room temperature. It forms at least five hydrates, several of which have been characterized crystallographically. These solids consist of the perchlorate anion linked via hydrogen bonds to H2O and H3O+ centers[6] Perchloric acid forms an azeotrope with water, consisting of about 72.5% perchloric acid. This form of the acid is stable indefinitely and is commercially available. Such solutions are hygroscopic. Thus, if left open to the air, concentrated perchloric acid dilutes itself by absorbing water from the air.
Dehydration of perchloric acid gives the anhydride dichlorine heptoxide, which is even more dangerous:[7]
- 2 HClO4 + P4O10 → Cl2O7 + "H2P4O11"
Uses
Perchloric acid is mainly produced as a precursor to ammonium perchlorate, which is used as rocket fuel. The growth in rocketry has led to increased production of perchloric acid. Several million kilograms are produced annually.[5] Perchloric acid is one of the most proven materials for etching of liquid crystal displays and critical electronics applications as well as ore extraction and has unique properties in analytical chemistry.[8] Additionally it is a useful component in etching of chrome[9]
As an acid
Perchloric acid, a superacid, is one of the strongest Brønsted-Lowry acids. Its pKa is −10.[10] It provides strong acidity with minimal interference because perchlorate is weakly nucleophilic (explaining the high acidity of HClO4). Other acids of noncoordinating anions, such as fluoroboric acid and hexafluorophosphoric acid are susceptible to hydrolysis, whereas perchloric acid is not. Despite hazards associated with the explosiveness of its salts, the acid is often preferred in certain syntheses.[11] For similar reasons, it is a useful eluent in ion-exchange chromatography.
It is also used for electropolishing/etching of aluminum, molybdenum, and other metals.
Safety
Given its strong oxidizing properties, perchloric acid is subject to extensive regulations.[12] It is highly reactive with metals (e.g., aluminium) and organic matter (wood, plastics). On February 20, 1947, in Los Angeles California, 17 people were killed and 150 injured when a bath, consisting of over 1000 litres of 75% perchloric acid and 25% acetic anhydride by volume, exploded. The plant, 25 other buildings and 40 automobiles were obliterated and 250 nearby homes were damaged. The bath was being used to electro-polish aluminum furniture. In addition, organic compounds were added to the overheating bath when an iron rack was replaced with one coated with cellulose acetobutyrate (Tenit-2 plastic). A few minutes later the bath exploded.[13][14]
Work conducted with perchloric acid must be conducted in fume hoods with a wash-down capability to prevent accumulation of oxidisers in the ductwork.
See also
References
- ↑ Samuel Fomon. Medicine and the Allied Sciences 1. p. 148.
- ↑ Safety data for concentrated perchloric acid, ca. 70% msds.chem.ox.ac.uk
- ↑ Handling of Perchloric acid ameslab.gov
- ↑ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 171. ISBN 978-0130399137.
- ↑ 5.0 5.1 Helmut Vogt, Jan Balej, John E. Bennett, Peter Wintzer, Saeed Akbar Sheikh, Patrizio Gallone "Chlorine Oxides and Chlorine Oxygen Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_483.
- ↑ Almlöf, Jan; Lundgren, Jan O.; Olovsson, Ivar "Hydrogen Bond Studies. XLV. Crystal structure of perchloric acid 2.5 hydrate" Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 1971, volume 27, pp. 898-904.doi:10.1107/S0567740871003236
- ↑ Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Translated by Mary Eagleson, William Brewer. San Diego: Academic Press. p. 464. ISBN 0-12-352651-5.
- ↑ http://www.gfschemicals.com/statics/coreproducts/Perchloric_acid.html
- ↑ http://engineering.dartmouth.edu/microeng/processing/etching/metal.etch.html
- ↑ Kathleen Sellers; Katherine Weeks; William R. Alsop; Stephen R. Clough; Marilyn Hoyt; Barbara Pugh (2006). Perchlorate: environmental problems and solutions. CRC Press. p. 16. ISBN 0-8493-8081-2.
- ↑ A. T. Balaban, C. D. Nenitzescu, K. Hafner and H. Kaiser (1973), "2,4,6-Trimethylpyrilium Perchlorate", Org. Synth.; Coll. Vol. 5: 1106
- ↑ Perchloric Acid, 60%, GR Material Safety Data Sheet Seton Resource Center
- ↑ R. C. Nester; G. F. Vander Voort (1992). Safety in the Metallographic Laboratory. ASTM Standardization News. p. 34.
- ↑ "CALIFORNIA: The Amazing Brew". Time.com. March 3, 1947.