Patchoulol
From Wikipedia, the free encyclopedia
| Patchoulol | ||
|---|---|---|
 | ||
 | ||
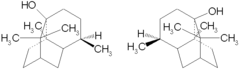
| IUPAC name 3,4,4αβ,5,6β,7,8,8α-Octahydro-4α,8αβ,9,9- | ||
| Other names Patchouli camphor; | ||
| Identifiers | ||
| CAS number | 5986-55-0 | |
| EC number | 227-807-2 | |
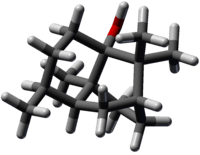
| Jmol-3D images | {{#if:O[C@@]23CC[C@@H]([C@@H]1C[C@@H](CC[C@@]12C)C3(C)C)C|Image 1 | |
| ||
| Properties | ||
| Molecular formula | C15H26O | |
| Molar mass | 222.36 | |
| Appearance | Hexagonal-trapezohedral crystals | |
| Density | 1.0284 g/mL | |
| Melting point | 56 °C, 329 K, 133 °F 39-40 °C (racemic) | |
| Boiling point | 140 °C; 284 °F; 413 K | |
| Solubility in water | practically insoluble | |
| Solubility in ethanol | soluble | |
| Solubility in diethyl ether | soluble | |
| Refractive index (nD) | 1.5029 | |
| Hazards | ||
| MSDS | External MSDS | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Patchoulol or patchouli alcohol (C15H26O) is a terpene extracted from Patchouli.[1] The (-)-optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol.
See also
References
- ↑ Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. (2006). "The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases". Archives of Biochemistry and Biophysics 454 (2): 123–136. doi:10.1016/j.abb.2006.08.006. PMID 16970904.
External links
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.