Partial oxidation
Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between thermal partial oxidation (TPOX) and catalytic partial oxidation (CPOX).
Principle
Partial oxidation is a technically mature process in which natural gas or a heavy hydrocarbon fuel (heating oil) is mixed with a limited amount of oxygen in an exothermic process.
- General reaction equation (without catalyst, TPOX):
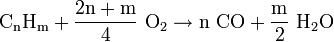 [1]
[1] - General reaction equation (with catalyst, CPOX):
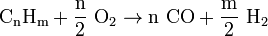
- Possible reaction equation (heating oil):
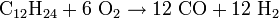
- Possible reaction equation (coal):

The formulas given for coal and heating oil show only a typical representative of these highly complex mixtures. Water is added to the process for getting both the extreme temperatures as well as extra control on the formation of soot.
TPOX
TPOX (thermal partial oxidation) reactions, which are dependent on the air-fuel ratio, proceed at temperatures of 1200°C and above.
CPOX
In CPOX (catalytic partial oxidation) the use of a catalyst reduces the required temperature to around 800°C – 900°C.
The choice of reforming technique depends on the sulfur content of the fuel being used. CPOX can be employed if the sulfur content is below 50 ppm. A higher sulfur content can poison the catalyst, so the TPOX procedure is used for such fuels. However, recent research shows that CPOX is possible with sulfur contents up to 400ppm.[2]
History
1926 – Vandeveer and Parr at the University of Illinois used oxygen to replace air.[3]
See also
- Hydrogen production
- Industrial gas
- IPOX (indirect partial oxidation)
- PROX
- Small stationary reformer
- Glossary of fuel cell terms
- Timeline of hydrogen technologies
References
- ↑ Rostrup-Nielsen, "Syngas in perspective", Catalysis Today 71 (2002), pp. 243-247.
- ↑ Electricity from wood through the combination of gasification and solid oxide fuel cells, Ph.D. Thesis by Florian Nagel, Swiss Federal Institute of Technology Zurich, 2008
- ↑ Industrial Gas Handbook, Frank G. Kerry, p. 230.
Source
- This article incorporates information from this version of the equivalent article on the German Wikipedia.