Paeonol
| Paeonol | |
|---|---|
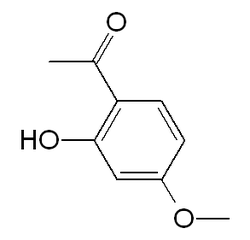 | |
| IUPAC name 1-(2-hydroxy-4-methoxyphenyl)ethanone | |
| Other names 2'-Hydroxy-4'-methoxyacetophenone; 1-(2-hydroxy-4-methoxyphenyl)ethan-1-one | |
| Identifiers | |
| CAS number | 552-41-0[1] |
| PubChem | 11092 |
| ChemSpider | 10621 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C9H10O3 |
| Molar mass | 166.18 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Paeonol is a phenolic compound found in peony such as Paeonia suffruticosa (moutan cortex),[2][3] in Arisaema erubescens[4] or in Dioscorea japonica.[5] It is a component found in many Traditional Chinese medicine remedies.[6]
Biological effects
Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer's disease.[7] Paeonol also reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats.[8]
It shows antimutagenic activities.[2][5] It also has anti-inflammatory and analgesic effects in carrageenan-evoked thermal hyperalgesia.[9] Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α.[10]
It has demonstrated significant MAOI activity. MAO-A and MAO-B inhibiting effects with IC50 values of 54.6 μM and 42.5 μM respectively.[11]
References
- ↑ http://www.chemblink.com/products/552-41-0.htm
- ↑ 2.0 2.1 Fukuhara Y and Yoshida D (1987). "Paeonol: a bio-antimutagen isolated from a crude drug, moutan cortex". Agricultural and biological chemistry 51 (5): 1441–1442. doi:10.1271/bbb1961.51.1441. INIST:7609719.
- ↑ Wu, Xinan; Chen, Hongli; Chen, Xingguo; Hu, Zhide (2003). "Determination of paeonol in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies following oral administration of Moutan cortex decoction". Biomedical Chromatography 17 (8): 504–8. doi:10.1002/bmc.259. PMID 14648606.
- ↑ Ducki S, Hadfield JA, Lawrence NJ, Xiuguo Zhang and McGown AT (1995). "Isolation of paeonol from Arisaema erubescens". Planta medica 61 (6): 586–587. doi:10.1055/s-2006-959390. PMID 8824957. INIST:2920867.
- ↑ 5.0 5.1 Miyazawa, Mitsuo; Shimamura, Hideo; Nakamura, Sei-Ichi; Kameoka, Hiromu (1996). "Antimutagenic Activity of (+)-β-Eudesmol and Paeonol fromDioscorea japonica". Journal of Agricultural and Food Chemistry 44 (7): 1647. doi:10.1021/jf950792u.
- ↑ Deng, Chunhui; Yao, Ning; Wang, Ben; Zhang, Xiangmin (2006). "Development of microwave-assisted extraction followed by headspace single-drop microextraction for fast determination of paeonol in traditional Chinese medicines". Journal of Chromatography A 1103 (1): 15–21. doi:10.1016/j.chroma.2005.11.023. PMID 16309693.
- ↑ Zhou, Jun; Zhou, Li; Hou, Deren; Tang, Jiaochun; Sun, Juanjuan; Bondy, Stephen C. (2011). "Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer's disease". Brain Research 1388: 141–7. doi:10.1016/j.brainres.2011.02.064. PMID 21377451.
- ↑ Hsieh, Ching-Liang; Cheng, Chin-Yi; Tsai, Tung-Hu; Lin, I-Hsin; Liu, Chung-Hsiang; Chiang, Su-Yin; Lin, Jaung-Geng; Lao, Chih-Jui et al. (2006). "Paeonol reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats". Journal of Ethnopharmacology 106 (2): 208–15. doi:10.1016/j.jep.2005.12.027. PMID 16458462.
- ↑ Chou, Tz-Chong (2003). "Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia". British Journal of Pharmacology 139 (6): 1146–52. doi:10.1038/sj.bjp.0705360. PMC 1573952. PMID 12871833.
- ↑ Kim, Sung Hoon; Kim, Seung-Ae; Park, Mi-Kyung; Kim, Seung-Hyung; Park, Young-Doo; Na, Ho-Jeong; Kim, Hyung-Min; Shin, Min-Kyu et al. (2004). "Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α". International Immunopharmacology 4 (2): 279–87. doi:10.1016/j.intimp.2003.12.013. PMID 14996419.
- ↑ Kong, L.D.; Cheng, Christopher H.K.; Tan, R.X. (2004). "Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones". Journal of Ethnopharmacology 91 (2–3): 351–355. doi:10.1016/j.jep.2004.01.013. PMID 15120460.