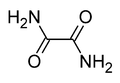
Oxamide
| Oxamide | ||
|---|---|---|
 |
 | |
| IUPAC name Ethanediamide | ||
| Other names Oxamide | ||
| Identifiers | ||
| CAS number | 471-46-5 | |
| Properties | ||
| Molecular formula | C2H4N2O2 | |
| Molar mass | 88.0654 g/mol | |
| Appearance | White powder | |
| Density | 1.667 g/cm3 | |
| Solubility in water | Soluble | |
| Solubility | ethanol | |
| Hazards | ||
| EU classification | Mild Irritant (6.1) | |
| R-phrases | R36 | |
| S-phrases | S25 | |
| Flash point | >300 °C | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Oxamide is the organic compound with the formula (CONH2)2. This white crystalline solid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid.
Production and applications
Oxamide is produced from hydrogen cyanide, which is oxidized to cyanogen, which is then hydrolyzed.[1]
The main application is as a substitute for urea in fertilizers. Oxamide hydrolyzes (releases ammonia) very slowly, which is sometimes preferred vs the quick release by urea.
It is used as a stabilizer for nitrocellulose preparations. It also finds use in APCP rocket motors as a high performance burn rate suppressant. The use of oxamide in concentrations of 1-3 wt% has shown to slow the linear burn rate while having minimal impact on propellant specific impulse.
Reactions
Upon heating above 350 °C, it decomposes to cyanogen and water. Oxamide derivatives form self-assembled monolayers consisting of a hydrogen bonded network.[2]
References
- ↑ Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic Acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a18_247.
- ↑ Nguyen T.L., Fowler F.W., Lauher J.W., "Commensurate and incommensurate hydrogen bonds. An exercise in crystal engineering." Journal of the American Chemical Society, 123(44), pp. 11057-64, 2001. doi:10.1021/ja016635v