Otolith
| Otolith | |
|---|---|
 | |
| Otolith organs showing detail of utricle, otoconia, endolymph, cupula, macula, hair cell filaments, and saccular nerve | |
 | |
| Juvenile herring. Length 30 mm; 3 months old; still transparent; the otoliths are visible left of the eye. | |
| Gray's | subject #232 1054 |
| MeSH | Otolithic+Membrane |
An otolith, (οτο-, oto-, ear + λιθος, lithos, a stone), also called statoconium or otoconium is a structure in the saccule or utricle of the inner ear, specifically in the vestibular labyrinth of vertebrates. The saccule and utricle, in turn, together make the otolith organs. They are sensitive to gravity and linear acceleration. Because of their orientation in the head, the utricle is sensitive to a change in horizontal movement, and the saccule gives information about vertical acceleration (such as when in an elevator).
Description
Endolymphatic infillings such as an otolith, (οτο-, oto-, ear + λιθος, lithos, a stone) or statoconia are structures in the saccule or utricle of the inner ear, specifically in the vestibular labyrinth of all vertebrates (fish, amphibians, reptiles, mammals and birds). In vertebrates the saccule and utricle together make the otolith organs. Both statoconia and otoliths are used as gravity, balance, movement, and directional indicators in all vertebrates and have a secondary function in sound detection in higher aquatic and terrestrial vertebrates.[1][2] They are sensitive to gravity and linear acceleration. Because of their orientation in the head, the utricle is sensitive to a change in horizontal movement, and the saccule gives information about vertical acceleration (such as when in an elevator).
Statoliths can be found in many invertebrate groups but are not contained in the structure of an inner ear. Mollusk statoliths are of a similar morphology to the displacement-sensitive organs of vertebrates;[3] however, the function of the mollusk statocyst is restricted to gravity detection and possibly some detection of angular momentum.[4] These are analogous structures, with similar form and function but not descended from a common structure.
Statoconia (also called otoconia) are numerous grains, often spherical in shape, between 1 and 50 µm; collectively, statoconia are also sometimes termed a statocyst. Otoliths (also called statoliths) are agglutinated crystals or crystals precipitated around a nucleus, with well defined morphology and together all may be termed endolymphatic infillings.[5][6][7]
Mechanism
The semicircular canals and sacs in all vertebrates are attached to endolymphatic ducts, which in some groups (such as sharks) end in small openings, called endolymphatic pores, on the dorsal surface of the head.[5] Extrinsic grains may enter through these openings, typically less than a millimeter in diameter. The size of material that enters is limited to sand-sized particles and in the case of sharks is bound together with endogenous organic matrix that the animal secretes.
In mammals, otoliths are small particles, composed of a combination of a gelatinous matrix and calcium carbonate in the viscous fluid of the saccule and utricle. The inertia of these small particles causes them to stimulate hair cells when the head moves. The hair cells are made up of 40 to 70 stereocilia and one hair cell, called the kinocilium, which is connected to an afferent nerve. When the body changes position or begins a movement the weight of the membrane bends the stereocilia and stimulates the hair cells. Hair cells send signals down sensory nerve fibers, which are interpreted by the brain as motion. The brain interprets the orientation of the head by comparing the input from the utricules and saccules from both ears to the input from the eyes, allowing the brain to discriminate a tilted head from movement of the entire body. When the head is in a normal upright position, the otolith presses on the sensory hair cell receptors. This pushes the hair cell processes down and prevents them from moving side to side. However, when the head is tilted, the pull of gravity on statoconia shift the hair cell processes to the side, distorting them and sending a message to the central nervous system that the head is no longer level but now tilted. (see: BPPV) This theory may have to be reevaluated because of an experiment in which a blindfolded owl in zero gravity was able to keep its head level while a handler was rocking its body back and forth.[8]
There is evidence that the vestibular system of mammals has retained some of its ancestral acoustic sensitivity and that this sensitivity is mediated by the otolithic organs (most likely the sacculus, due to its anatomical location). In mice lacking the otoconia of the utricle and saccule, this retained acoustic sensitivity is lost.[2] In humans vestibular evoked myogenic potentials occur in response to loud, low frequency acoustic stimulation in patients with sensioneural hearing loss.[1] Vestibular sensitivity to ultrasonic sounds has also been hypothesised to be involved in the perception of speech presented at artificially high frequencies, above the range of the human cochlea (~18 kHz).[9] In mice sensation of acoustic information via the vestibular system has been demonstrated to have a behaviourally relevant effect; response to an elicited acoustic startle reflex is larger in the presence of loud, low frequency sounds that are below the threshold for the mouse cochlea (~4 Hz), raising the possibility that the acoustic sensitivity of the vestibular system may extend the hearing range of small mammals.[2]
Paleontology
After the death and decomposition of a fish, otoliths and statoconia may be preserved within the body of an organism or be dispersed before burial and fossilization. Dispersed otoliths are one of the many microfossils which can be found though a micropalaeontological analysis of a fine sediment. Their stratigraphic significance is minimal, but can still be used to characterize a level or interval. Fossil otoliths are rarely found in situ (on the remains of the animal), likely because they are not recognized separately from the surrounding rock matrix. In some cases, due to differences in colour, grain size, or a distinctive shape, they can be identified. These rare cases are of special significance, since the presence, composition, and morphology of the material can clarify the relationship of species and groups, as in the case of lineages of primitive fish which shows that endolymphatic infillings were similar in elemental composition to the rock matrix but were restricted to coarse grained material, which presumably is better for the detection of gravity, displacement, and sound. The presence of these extrinsic grains, in osteostracans, chondrichthyans, and acanthodians indicates a common inner ear physiology and presence of open endolymphatic ducts.[5]
Ecology
Composition
The composition of fish otoliths is also proving useful to fisheries scientists. The calcium carbonate that the otolith is composed of is primarily derived from the water. As the otolith grows, new calcium carbonate, usually aragonite but sometimes vaterite, crystals form. As with any crystal structure, lattice vacancies will exist during crystal formation allowing trace elements from the water to bind with the otolith. Studying the trace elemental composition or isotopic signatures of trace elements within a fish otolith gives insight to the water bodies fish have previously occupied. The most studied trace and isotopic signatures are strontium due to the same charge and similar ionic radius to calcium; however, scientists can study multiple trace elements within an otolith to discriminate more specific signatures. A common tool used to measure trace elements in an otolith is a laser ablation inductively coupled plasma mass spectrometer. This tool can measure a variety of trace elements simultaneously. A secondary ion mass spectrometer can also be used. This instrument can allow for greater chemical resolution but can only measure one trace element at a time. The hope of this research is to provide scientists with valuable information on where fish have traveled. Combined with otolith annuli, scientists can add how old fish were when they traveled through different water bodies. All this information can be used to determine fish life cycles so that fisheries scientists can make better informed decisions about fish stocks.
Growth rate and age

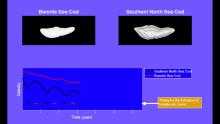
Finfish (class Osteichthyes) have three pairs of otoliths – the sagittae (singular sagitta), lapilli (singular lapillus), and asterisci (singular asteriscus). The sagittae are largest, found just behind the eyes and approximately level with them vertically. The lapilli and asterisci (smallest of the three) are located within the semicircular canals.
The shapes and proportional sizes of the otoliths vary with fish species. In general, fish from highly structured habitats such as reefs or rocky bottoms (e.g. snappers, groupers, many drums and croakers) will have larger otoliths than fish that spend most of their time swimming at high speed in straight lines in the open ocean (e.g. tuna, mackerel, dolphinfish). Flying fish have unusually large otoliths, possibly due to their need for balance when launching themselves out of the water to "fly" in the air. Often, the fish species can be identified from distinct morphological characteristics of an isolated otolith.
Fish otoliths accrete layers of calcium carbonate and gelatinous matrix throughout their lives. The accretion rate varies with growth of the fish – often less growth in winter and more in summer – which results in the appearance of rings that resemble tree rings. By counting the rings, it is possible to determine the age of the fish in years.[10] Typically the sagitta is used, as it is largest,[11] but sometimes lapilli are used if they have a more convenient shape. The asteriscus, which is smallest of the three, is rarely used in age and growth studies.
In addition, in most species the accretion of calcium carbonate and gelatinous matrix alternates on a daily cycle. It is therefore also possible to determine fish age in days. This latter information is often obtained under a microscope, and provides significant data to early life history studies.
By measuring the thickness of individual rings, it has been assumed (at least in some species) to estimate fish growth because fish growth is directly proportional to otolith growth. However, some studies disprove a direct link between body growth and otolith growth. At times of lower or zero body growth the otolith continues to accrete leading some researchers to believe the direct link is to metabolism, not growth per se. Otoliths, unlike scales, do not reabsorb during times of decreased energy making it even more useful tool to age a fish. Fish never stop growing entirely, though growth rate in mature fish is reduced. Rings corresponding to later parts of the life cycle tend to be closer together as a result.
Age and growth studies of fish are important for understanding such things as timing and magnitude of spawning, recruitment and habitat use, larval and juvenile duration, and population age structure. Such knowledge is in turn important for designing appropriate fisheries management policies.
Diet research
Since the compounds in fish otoliths are resistant to digestion, they are found in the digestive tracts and scats of piscivorous marine mammals, such as dolphins, seals, sea lions and walruses. Many fish can be identified to genus and species by their sagittal otoliths. Otoliths can therefore, to some extent, be used to reconstruct the prey composition of marine mammal diets.
Sagittal otoliths (sagittae) are bilaterally symmetrical, with each fish having one right and one left. Separating recovered otoliths into right and left, therefore, allows one to infer a minimum number of prey individuals ingested for a given fish species. Otolith size is also proportional to the length and weight of a fish. They can therefore be used to back-calculate prey size and biomass, useful when trying to estimate marine mammal prey consumption, and potential impacts on fish stocks.[12]
Otoliths cannot be used alone to reliably estimate cetacean or pinniped diets, however. They may suffer partial or complete erosion in the digestive tract, skewing measurements of prey number and biomass.[13] Species with fragile, easily digested otoliths may be underestimated in the diet. To address these biases, otolith correction factors have been developed through captive feeding experiments, in which seals are fed fish of known size, and the degree of otolith erosion is quantified for different prey taxa.[14]
The inclusion of fish vertebrae, jaw bones, teeth, and other informative skeletal elements improves prey identification and quantification over otolith analysis alone.[15] This is especially true for fish species with fragile otoliths, but other distinctive bones, such as Atlantic mackerel (Scomber scombrus), and Atlantic herring (Clupea harengus).[16]
See also
References
| Wikimedia Commons has media related to Otolith. |
- ↑ 1.0 1.1 Sheykholeslami, Kianoush; Kaga, Kimitaka (2002). "The otolithic organ as a receptor of vestibular hearing revealed by vestibular-evoked myogenic potentials in patients with inner ear anomalies". Hearing Research 165 (1–2): 62–67. doi:10.1016/S0378-5955(02)00278-2. ISSN 0378-5955. PMID 12031516.
- ↑ 2.0 2.1 2.2 Jones, Gareth P.; Lukashkina, Victoria A.; Russell, Ian J.; Lukashkin, Andrei N. (2010). "The Vestibular System Mediates Sensation of Low-Frequency Sounds in Mice". Journal of the Association for Research in Otolaryngology 11 (4): 725–732. doi:10.1007/s10162-010-0230-7. ISSN 1525-3961. PMC 2975890. PMID 20821033.
- ↑ Gauldie, R. W. 1993. Polymorphic structure of fish otoliths. Journal of Morphology 218:1–28.
- ↑ Wolff, H. C. 1973. Multi-directional sensitivity of statocyst receptor cells of the opisthobranch gastropod Aplysia limacina. Marine Behavior and Physiology 1:361–373.
- ↑ 5.0 5.1 5.2 Sahney, S. and M.V.H. Wilson 2001. Extrinsic labyrinth infillings imply open endolymphatic ducts in Lower Devonian osteostracans, acanthodians, and putative chondrichthyans. Journal of Vertebrate Paleontology 21:660-669.
- ↑ Nolf, D. 1985. Otolithi Piscium; in H.-P. Schultze (ed.), Handbook of Paleoichthyology, Vol. 10. Gustav Fischer Verlag, Stuttgart, 145pp.
- ↑ Schultze, H.-P. 1990. A new acanthodian from the Pennsylvanian of Utah, U.S.A. and the distribution of otoliths in gnathostomes. Journal of Vertebrate Paleontology 10:49–58.
- ↑ Skylab Archives, 1984.
- ↑ Lenhardt, M.; Skellett, R; Wang, P; Clarke, A. (1991). "Human ultrasonic speech perception". Science 253 (5015): 82–85. doi:10.1126/science.2063208. ISSN 0036-8075. PMID 2063208.
- ↑ Quality control of age data at the Alaska Fisheries Science Center (PDF) Daniel K. Kimura, Delsa M. Anderl, Marine and Freshwater Research, 2005, 56, 783–789. Retrieved 2007-04-07.
- ↑ Fish Age and Growth with Otoliths Tennessee Wildlife Resources Agency. Retrieved 2007-04-07.
- ↑ Arim, M. and D.E. Naya. 2003. Pinniped diets inferred from scats: analysis of biases in prey occurrence. Can. J. Zool. 81: 67-73.
- ↑ Bowen, W.D. 2000. Reconstruction of pinniped diets: accounting for complete digestion of otoliths and cephalopod beaks. Can. J. Fish. Aquat. Sci. 57(5): 898-905
- ↑ Grellier, K. and P. S. Hammond. 2005. Feeding method affects otolith digestion in captive gray seals: Implications for diet composition estimation. Marine Mammal Science 21(2): 296–306
- ↑ 04 Browne FISH BULL 100(3)
- ↑ Ouwehand, J., M. F. Leopold, et al. (2004). "A Comparative Study Of The Diet Of Guillemots Uria Aalge And Razorbills Alca Torda Killed During The Tricolor Oil Incident In The South-Eastern North Sea In January 2003." Atlantic Seabirds 6(3): 147-163.
| ||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
