Organoxenon compound
Organoxenon compounds in organic chemistry contain carbon to xenon chemical bonds. The first organoxenon compounds were divalent, such as (C6F5)2Xe. The first tetravalent organoxenon compound, [C6F5XeF2][BF4], was synthesized in 2004.[1] So far, more than one hundred organoxenon compounds have been researched.
Most of the organoxenon compounds are more unstable than xenon fluorides due to the high polarity. The molecular dipoles of xenon difluoride and xenon tetrafluoride are both 0 D. The early synthesized ones only contain perfluoro groups, but later some other groups were found, e.g. 2,4,6-trifluorophenyl.[2]
Xe(II)
The most common bivalent organoxenon compound is C6F5XeF, which is always used as a precursor to other organoxenon compounds. Due to the unstability of xenon fluoride, it is impossible to synthesize organoxenon compound by using general organic reagents. Most frequently used fluorinating agents include Cd(ArF)2(subscript "F" means fluorine-including aryl), C6F5SiF3, and C6F5SiMe3 (should be used along with fluoride).
With the use of stronger Lewis acids, such as C6F5BF2, ionic compounds like [RXe][ArFBF3] can be produced. Alkenyl and alkyl organoxenon compounds are prepared in this way as well, for example, C6F5XeCF=CF2 and C6F5XeCF3.[2]
Some typical reactions are listed below:
The third reaction also produces (C6F5)2Xe, Xe(2,4,6-C6H2F3)2 and so on.
The precursor C6F5XeF can be prepared by the reaction of trimethyl(pentaflurophenyl)silane(C6F5SiMe3) and xenon difluoride. Adding fluoride to the adduct of C6F5XeF and arsenic pentafluoride is another method.[2]
Xe(IV)
In 2000, Karel Lutar and Boris Žemva et al. produced an ionic compound. They treated xenon tetrafluoride and difluoro(pentaflurophenyl)borane in dichloromethane at -55°C:
The compound is an extremely strong fluorinating agent, and it is capable of converting (C6F5)3P to (C6F5)3PF2, C6F5I to C6F5IF2, even iodine to iodine pentafluoride.[1]
See also
- Compounds of carbon with other elements in the periodic table:
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ 1.0 1.1 "The First Organoxenon(IV) Compound: Pentafluorophenyldifluoroxenonium(IV) Tetrafluoroborate". Angewandte Chemie International Edition 39 (2): 391–393. 2000-01-16. doi:10.1002/(SICI)1521-3773(20000117)39:2<391::AID-ANIE391>3.0.CO;2-U.
|coauthors=requires|author=(help) - ↑ 2.0 2.1 2.2 FROHN, H (2004-05-31). "C6F5XeF, a versatile starting material in xenon-carbon chemistry". Journal of Fluorine Chemistry 125 (6): 981–988. doi:10.1016/j.jfluchem.2004.01.019.
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
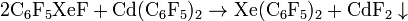


![{\rm {\ XeF_{4}+C_{6}F_{5}BF_{2}{\xrightarrow[ {-55^{o}C}]{CH_{2}Cl_{2}}}[C_{6}F_{5}XeF_{2}]^{+}BF_{4}^{-}}}](/2014-wikipedia_en_all_02_2014/I/media/5/c/c/5/5cc5c1778c48a339a34087dd52150ca7.png)