Organotin chemistry

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry.[1] The first organotin compound was diethyltin diiodide ((C2H5)2SnI2 , ref to. Tin), discovered by Edward Frankland in 1849. An organotin compound is commercially applied as a hydrochloric acid scavenger (or heat stabilizer) in polyvinyl chloride and as a biocide. Tributyltin oxide has been extensively used as a wood preservative. Tributyltin compounds are used as marine anti-biofouling agents. Concerns over toxicity[2] of these compounds (some reports describe biological effects to marine life at a concentration of 1 nanogram per liter) have led to a worldwide ban by the International Maritime Organization. n-Butyltin trichloride is used in the production of tin dioxide layers on glass bottles by chemical vapor deposition.
Structure of organotin compounds
Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful.
Organic derivatives of tin(IV)
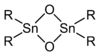
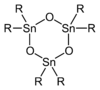
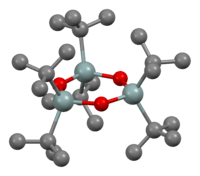
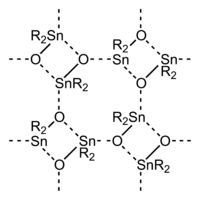
The entire series R4−nSnCln are known for many R groups and values of n up to 4. Analogous derivatives are known for other halides. Alkoxide and carboxylate derivatives however tend to associate and often poorly characterized. The oxides are also complicated. Many diorganotin oxides, (R2SnO)n are oligomeric:
With bulky R groups, diorganotin oxides adopt cyclic trimeric or dimeric structures consisting of Sn3O3 and Sn2O2 rings, respectively.
 |  |  |  |
| | |
Hypercoordinated stannanes
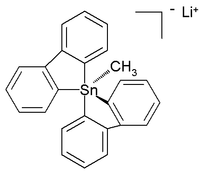
Unlike their carbon(IV) analogues but like silicon compounds, tin(IV) can also be coordinated to five and even six atoms instead of the regular four. These hypercoordinated compounds usually have electronegative substituents. Lithium pentaorganostannates were first detected and characterized in solution in 1986,[3] while in the subsequent year a six-coordinated tetraorganotin compound was reported.[4] In 2007 a crystal structure of room-temperature stable (in argon) all-carbon pentaorganostannane was reported as the lithium salt with this structure:[5]
In this distorted trigonal bipyramidal structure the carbon to tin bond lengths (2.26 Å apical, 2.17 Å equatorial) are larger than regular C-Sn bonds (2.14 Å) reflecting its hypervalent nature.
Tin radicals (organic derivatives of tin(III))
Tin radicals, with the formula R3Sn, are called stannyl radicals.[6] They are invoked as intermediates in certain atom-transfer reactions. For example, tributyltin hydride (tri-n-butylstannane) serves as a good source of "hydrogen atoms" because of the stability of the tributytin radical.[7]
Organic derivatives of tin(II)
Organotin(II) compounds are somewhat rare. Compounds with the empirical formula SnR2 are somewhat fragile and exist as rings or polymers when R is not bulky. The polymers are called polystannanes and have the formula (SnR2)n.
In principle divalent tin compounds might be expected to form analogues of alkenes with a formal double bond. Indeed compounds with the formula Sn2R4, called distannenes, are known for certain organic substituents. The Sn centres tend to be highly pyramidal. Monomeric compounds with the formula SnR2, analogues of carbenes are also known in a few cases. One example is [Sn(SiR3]2 where R = CH(SiMe3)2 (Me = methyl). Such species reversibly dimerize to the distannylene upon crystallization:[8]
- 2 R2Sn
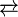 (R2Sn)2
(R2Sn)2
Stannenes, compounds with tin–carbon double bonds, are exemplified by derivatives of stannabenzene. Stannoles, structural analogs of cyclopentadiene, exhibit little C-Sn double bond character.
Organic derivatives of tin(I)
Compounds of Sn(I) are rare and only observed with very bulky ligands. One prominent family of cages is accessed by pyrolysis of the 2,6-diethylphenyl-substituted tristannylene [Sn(C6H3-2,6-Et2)2]3, which affords the cubane and a prismane. These cages contain Sn(I) and have the formula [Sn(C6H3-2,6-Et2)]n where n = 8, 10.[9] A stannyne contains a carbon to tin triple bond and a distannyne a triple bond between two tin atoms (RSnSnR). Distannynes only exist for extremely bulky substituents. Unlike alkynes, the C-Sn-Sn-C core of these distannynes are nonlinear, although they are planar. The Sn-Sn distance is 3.066(1) Å, and the Sn-Sn-C angles are 99.25(14)°. Such compounds are prepared by reduction of bulky aryltin(II) halides.[10]

Preparation of organotin compounds
Organotin compounds can be synthesised by reaction of a Grignard reagent with tin halides for example tin tetrachloride. An example is the organic synthesis of tributyl-[(Z)-5-phenyl-2-penten-2-yl]stannane:[11][12]
The Wurtz-like coupling of alkyl sodium compounds with tin halides yield tetraorganotin compounds. Another method is an redistribution reaction of tin halides with organoaluminium compounds (AlR3). Triorganotin halides can be prepared in the Kocheshkov redistribution reaction.
Reactions of organotin compounds
Important reactions involving organotin compounds are the Stille reaction (coupling reaction with sp2-hybridized organic halides catalyzed by palladium):
and organostannane additions (nucleophilic addition of an allyl-, allenyl-, or propargylstannanes to an aldehydes and imines). Organotin compounds are also used extensively in radical chemistry (e.g. radical cyclizations, Barton–McCombie deoxygenation, Barton decarboxylation, etc.).
Use and toxicity
- Tetraorganotins are very stable molecules with low toxicity and low biological activity. They are unusable as biocides, but they can be metabolized to toxic triorganotin compounds. They are used as starting materials for catalysts.
- Triorganotins are very toxic. Tri-n-alkyltins are phytotoxic and therefore cannot be used in agriculture. Depending on the organic groups, they can be powerful bactericides and fungicides. Tributyltins are used as industrial biocides, e.g. as antifungal agents in textiles and paper, wood pulp and paper mill systems, breweries, and industrial cooling systems. Tributyltins are also used in marine anti-fouling paint. Triphenyltins are used as active components of antifungal paints and agricultural fungicides. Other triorganotins are used as miticides and acaricides.
- Diorganotins have no antifungal activity, low toxicity, and low antibacterial activity, except for diphenyltins. They are used in polymer manufacturing, as PVC heat stabilizers, catalysts, in the manufacturing of polyurethane and silicone curing. DBT is however immunotoxic, and a recent paper suggests a link to auto-immune related diseases.[13]
- Monoorganotins have no biocidal activity and their toxicity to mammals is very low. Methyltin, butyltin, octyltin and monoestertins are used as PVC heat stabilizers.
- Many different organotin complexes are being studied in anticancer therapy, observing that their cytotoxicity and selectivity towards cancer cell is higher than that of cisplatin.[14]
Compounds
Organotin compounds are used commercially in a wide range of applications such as biocides, insecticides, chemical intermediates and as catalysts.
- Organotin compounds
-

Tetrabutyltin starting material for the di- and tributyl compounds
-

Tributyltin oxide, a colorless to pale yellow liquid used in wood preservation
-
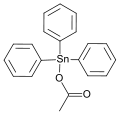
Triphenyltin acetate, an off-white crystalline solid, used as an insecticide and a fungicide
-

Triphenyltin chloride, a white crystalline solid, used as a biocide and an intermediate in chemical synthesis
-

Trimethyltin chloride also a biocide
-

Triphenyltin hydroxide, an off-white powder, used as a fungicide and to sterilize insects
-

Azocyclotin, a colorless crystalline solid, used as a long-acting acaricide for control of spider mites on plants
-
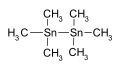
Hexamethylditin used as an intermediate in chemical synthesis
-

Tetraethyltin, boiling point 63–65° /12 mm is a catalyst[1]
Cite error: There are <ref> tags on this page, but the references will not show without a {{reflist}} template (see the help page).
See also
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ Sander H.L. Thoonen, Berth-Jan Deelman, Gerard van Koten (2004). "Synthetic aspects of tetraorganotins and organotin(IV) halides". Journal of Organometallic Chemistry (689): 2145–2157.
- ↑ Gajda, M.; Jancso, A. (2010). "Organotins, formation, use, speciation and toxicology". Metal ions in life sciences (Cambridge: RSC publishing). 7, Organometallics in environment and toxicology. ISBN 9781847551771.
- ↑ Reich, Hans J.; Phillips, Nancy H. (1986). "Lithium-Metalloid Exchange Reactions. Observation of Lithium Pentaalkyl/aryl Tin Ate Complexes". J. Am. Chem. Soc. 108: 2102. doi:10.1021/ja00268a067.
- ↑ V. G. Kumar Das, Lo Kong Mun, Chen Wei, and Thomas C. W. Mak (1987). "Synthesis, Spectroscopic Study, and X-ray Crystal Structure of Bis[3-(2-pyridyl)-2-thienyl-C,N]diphenyltin(IV): The First Example of a Six-Coordinate Tetraorganotin Compound". Organometallics 6: 10. doi:10.1021/om00144a003.
- ↑ Masaichi Saito, Sanae Imaizumi, Tomoyuki Tajima, Kazuya Ishimura, and Shigeru Nagase (2007). "Synthesis and Structure of Pentaorganostannate Having Five Carbon Substituents". J. Am. Chem. Soc. 129: 10974–10975. doi:10.1021/ja072478.
- ↑ Organotin chemistry 2004 Alwyn George Davies ISBN 3-527-31023-1
- ↑ T. V. RajanBabu, P. C. B. Page B. R. Buckley "Tri-n-butylstannane" in e-EROS Encyclopedia of Reagents for Organic Synthesis, 2004. doi:10.1002/047084289X.rt181.pub2
- ↑ Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5
- ↑ Lawrence R. Sita "Heavy-Metal Organic Chemistry: Building with Tin" Acc. Chem. Res., 1994, volume 27, pp 191–197. doi: 10.1021/ar00043a002
- ↑ Philip P. Power "Bonding and Reactivity of Heavier Group 14 Element Alkyne Analogues" Organometallics 2007, volume 26, pp 4362–4372. doi:10.1021/om700365p
- ↑ Martin J. Stoermer, John T. Pinhey (1998). "Tributyl-[(Z)-5-phenyl-2-penten-2-yl]stannane". Molecules 3: M67.
- ↑ A Grignard reagent is prepared from magnesium turnings and (Z)-2-bromo-5-phenyl-2-pentene in dry tetrahydrofuran and titrated with tributyltin chloride until the solution decolourises. The resulting solution is stirred at room temperature for 1 hour and the solvent is removed in a rotavapor. Diethyl ether is added and the ether extract is washed with brine and filtered and the ether evaporates in a rotavapor. The crude product is kugelrohr distilled to yield tributyl-[(Z)-5-phenyl-2-penten-2-yl]stannane as a colourless oil.
- ↑ C Gumy et al. (2008). "Dibutyltin Disrupts Glucocorticoid Receptor Function and Impairs Glucocorticoid-Induced Suppression of Cytokine Production". PLoS ONE 3: e3545. Bibcode:2008PLoSO...3.3545G. doi:10.1371/journal.pone.0003545.
- ↑ S. Gómez-Ruiz et al. (2008). "Study of the cytotoxic activity of di and triphenyltin(IV) carboxylate complexes". Journal of Inorganic Biochemistry 102 (12): 2087. doi:10.1016/j.jinorgbio.2008.07.009. PMID 18760840.
External links
- National Pollutant Inventory Fact Sheet for organotins
- Industry information site
- Organotin chemistry in synthesis
- EU bans certain organotin compounds in consumer products
| ||||||||||||||||||||