Opioid
An opioid is any psychoactive chemical that resembles morphine or other opiates in its pharmacological effects. Opioids work by binding to opioid receptors, which are found principally in the central and peripheral nervous system and the gastrointestinal tract. The receptors in these organ systems mediate both the beneficial effects and the side effects of opioids.
Although the term opiate is often used as a synonym for opioid, the term opiate is properly limited to the natural alkaloids found in the resin of the opium poppy (Papaver somniferum), while opioid refers to both opiates and synthetic substances, as well as to opioid peptides.
Opioids are among the world's oldest known drugs; the therapeutic use of the opium poppy predates recorded history. The analgesic (painkiller) effects of opioids are due to decreased perception of pain, decreased reaction to pain as well as increased pain tolerance. The side effects of opioids include sedation, respiratory depression, constipation, and a strong sense of euphoria. Opioids can cause cough suppression, which can be both an indication for opioid administration or an unintended side effect. Opioid dependence can develop with ongoing administration, leading to a withdrawal syndrome with abrupt discontinuation. Opioids are not only well known for their addictive properties, but also for their ability to produce a feeling of euphoria, motivating some to use opioids recreationally.
Medical uses
Opioids have long been used to treat acute pain (such as post-operative pain).[1] They have also been found to be invaluable in palliative care to alleviate the severe, chronic, disabling pain of terminal conditions such as cancer, and degenerative conditions such as rheumatoid arthritis. In many cases opioids are a successful long-term care strategy for those in chronic cancer pain. However, opioids should be used cautiously in chronic non-cancer pain.[2]
Opioids for pain relief are also used when nondrug pain treatment options including cognitive behavioral therapy, exercise, spinal manipulation, and physical medicine and rehabilitation programs are insufficient to meet therapy goals.[3] Patients taking opioids talk with their health care provider to develop a personal health plan which includes a combination of therapies, perhaps including drug and nondrug treatments, to relieve pain.[3]
In treating chronic pain, opioids are an option to be tried after other less risky pain relievers have been considered, including paracetamol/acetaminophen or NSAIDs like ibuprofen or naproxen.[3][4] Some types of chronic pain, including the pain caused by fibromyalgia or migraine, are preferentially treated with drugs other than opioids.[3][5][6] The efficacy of using opioids to lessen chronic neuropathic pain is uncertain.[7]
Opioids such as Nalbuphine have also been used to relieve labor pain during Childbirth.[8]
Contraindications
Opioids are contraindicated as a first-line treatment for headache because they impair alertness, bring risk of dependence and addiction, and increase the risk that episodic headaches will become chronic.[9] Opioids also can cause heightened sensitivity to headache pain.[9] When other treatments fail or are unavailable, opioids may be appropriate for treating headache if the patient can be monitored to prevent the development of chronic headache.[9]
Opioids are sometimes used in the management of non-malignant chronic pain. This practice has now led to a new and growing problem with addiction and misuse of opioids.[2] Because of various negative effects the use of opioids for long term management of chronic pain is not indicated unless other less risky pain relievers have been found ineffective.[3] Chronic pain which occurs only periodically, such as that from nerve pain, migraines, and fibromyalgia, frequently is better treated with drugs other than opioids.[3][5] Acetaminophen and non-steroidal anti-inflammatory drugs including ibuprofen and naproxen are common and safer alternatives or complements to opioids.[3]
Safety
Studies have shown opioids to be safe when they are used correctly and in the ways that are well understood.[10]
Carefully titrating the dose of opioids can provide for effective pain relief while minimizing adverse effects. Morphine and diamorphine have been shown to have a wider therapeutic range or "safety margin" than some other opioids. It is impossible to tell which patients need low doses and which need high doses, so all have to be started on low doses, unless changing from another strong opioid.[11]
Opioid analgesics do not cause any specific organ toxicity, unlike many other drugs, such as aspirin and acetaminophen. They are not associated with upper gastrointestinal bleeding and renal toxicity.[12]
In older adults, opioid use is associated with increased adverse effects such as "sedation, nausea, vomiting, constipation, urinary retention, and falls".[13] As a result older adults taking opioids are at greater risk for injury.[14]
There are gaps in available research describing the safe use of opioids long-term or comparing the relative safety of the long-term use of various opioids to each other.[3] The research also does not have information about the extent to which opioids differ in terms of the risk they bring for causing addiction.[3]
Research suggests that when methadone is used long-term use it can build-up unpredictably in the body and lead to potentially deadly slowed breathing.[3][15][16] Regular physician monitoring reduces the likelihood of problems.[3][15]
According to a cohort study, the rate of opioid related death was 0.017% per year amongst patients prescribed opioids for non-cancer pain from 1997-2005 in Washington State. Increasing dose and age were found to correlate with increased risk of overdose.[17] While a cohort study is a higher level of evidence than case-control, a case-control study done in Canada correlates well as it had an opioid related death rate of 0.024% per year amongst patients prescribed opioids for non-cancer pain over a 10-year period.[18]
Tolerance
Tolerance to opioids may occur. In spite of tolerance, the dose required to achieve analgesia can level off for many months at a time, depending on severity of pain, which varies.[citation needed]
High doses are not necessarily required to control the pain of advanced or end-stage disease.[citation needed]
Tolerance is a process characterized by neuroadaptations that result in reduced drug effects. While receptor downregulation may often play an important role other mechanisms are also known.[19] Tolerance is more pronounced for some effects than for others; tolerance occurs slowly to the effects on mood, itching, urinary retention, and respiratory depression, but occurs more quickly to the analgesia and other physical side effects. However, tolerance does not develop to constipation or miosis (the constriction of the pupil of the eye to less than or equal to two millimeters). This idea has been challenged, however, with some authors arguing that tolerance does develop to miosis.[20]
Tolerance to opioids is attenuated by a number of substances, including:
- NMDA antagonists, such as dextromethorphan, ketamine,[25] and memantine.[26]
- Newer agents such as the phosphodiesterase inhibitor ibudilast have also been researched for this application.[30]
Tolerance is a physiologic process where the body adjusts to a medication that is frequently present, usually requiring higher doses of the same medication over time to achieve the same effect. It is a common occurrence in individuals taking high doses of opioids for extended periods, but does not predict any relationship to misuse or addiction.
Dependence is characterised by unpleasant withdrawal symptoms that occur if opioid use is abruptly discontinued. The withdrawal symptoms for opiates include severe dysphoria, craving for another opiate dose, irritability, sweating, nausea, rhinorrea, tremor, vomiting and myalgia. Slowly reducing the intake of opioids over days and weeks will reduce or eliminate the withdrawal symptoms.[11] The speed and severity of withdrawal depends on the half-life of the opioid; heroin and morphine withdrawal occur more quickly and are more severe than methadone withdrawal. The acute withdrawal phase is often followed by a protracted phase of depression and insomnia that can last for months. The symptoms of opioid withdrawal can be treated with other medications, such as clonidine.[31] Physical dependence does not predict drug misuse or true addiction, and is closely related to the same mechanism as tolerance.
Addiction
Addiction is the process whereby physical and psychological dependence develops to any drug—including opioids. This tends to occur over time or with higher doses of substances. Withdrawal symptoms can reinforce the addiction, driving the user to continue taking the drug despite negative consequences of behavior. Psychological addiction is more common in people insufflating or injecting opioids recreationally, rather than taking them orally as prescribed for medical reasons.[11]
In European nations such as Austria, Bulgaria, and Slovakia, slow release oral morphine formulations are used in opiate substitution therapy for patients who do not well tolerate the side effects of buprenorphine or methadone. In other European countries including the UK, this is also legally used for OST although on a varying scale of acceptance.
Tamper-release formulations of time-controlled preparations of medications are intended to curb abuse and addiction rates while trying to still provide legitimate pain relief and ease of use to pain patients. Questions remain, however, about the efficacy and safety of these types of preparations. Further tamper resistant medications are currently under consideration with trials for market approval by the FDA.[32][33]
Adverse effects
- Common and short term
- Other
- Opioid dependence
- Dizziness
- Decreased sex drive
- impaired sexual function
- Decreased testosterone levels
- Depression
- Immunodeficiency
- opioid-induced abnormal pain sensitivity
- Irregular menstruation
- Increased risk of falls
- Slowed breathing
The amount of evidence available only permits making a weak conclusion, but it suggests that a physician properly managing opioid use in patients with no history of substance dependence or substance abuse can can give long-term pain relief with little risk of developing addiction, abuse, or other serious side effects.[10]
Problems with opioids include the following:
- Some people find that opioids do not relieve all of their pain.[3][35]
- Some people find that opioids side effects cause problems which outweigh the therapy's benefit[3][10]
- Some people build tolerance to opioids over time. This requires them to increase their drug dosage to maintain the benefit, and that in turn also increases the unwanted side effects.[3][10]
- Long-term opioid use can cause opioid-induced hyperalgesia, which is a condition in which the patient has increased sensitivity to pain.[3][36]
All of the opioids can cause side effects.[3][34] Common adverse reactions in patients taking opioids for pain relief include nausea and vomiting, drowsiness, itching, dry mouth, dizziness, and constipation.[3][11][34]
When prescribing an opioid, physicians have a process to recommend the correct one, find the right dosage for the patient, and then minimize the side effects.[3]
Nausea
Tolerance to nausea occurs within 7–10 days, during which antiemetics (e.g. low dose haloperidol once at night) are very effective.[citation needed] Due to severe side effects such as tardive dyskinesia, haloperidol is currently rarely used. A related drug, Compazine (prochlorperazine) is more often used, although it has similar risks. Stronger antiemetics such as ondansetron or tropisetron may be indicated if nausea is severe or continues for an extended period, although these tend to be avoided due to their high cost unless nausea is really problematic. A cheaper alternative is dopamine antagonists, e.g. domperidone and metoclopramide. Domperidone does not cross the blood–brain barrier, so blocks opioid emetic action in the chemoreceptor trigger zone without adverse central anti-dopaminergic effects (not available in the U.S.) Some antihistamines with anti-cholinergic properties (e.g. orphenadrine or diphenhydramine) may also be effective. The first-generation anti-histamine hydroxyzine is very commonly used, with the added advantages of not causing movement disorders, and also possessing analgesic-sparing properties.
- 5-HT3 antagonists (e.g. ondansetron)
- Dopamine antagonists (e.g. domperidone)
- Anti-cholinergic antihistamines (e.g. diphenhydramine)
Vomiting
Vomiting is due to gastric stasis (large volume vomiting, brief nausea relieved by vomiting, oesophageal reflux, epigastric fullness, early satiation), besides direct action on the chemoreceptor trigger zone of the area postrema, the vomiting centre of the brain. Vomiting can thus be prevented by prokinetic agents (e.g. domperidone or metoclopramide 10 mg every eight hours). If vomiting has already started, these drugs need to be administered by a non-oral route (e.g. subcutaneous for metoclopramide, rectally for domperidone).
- Prokinetic agents (e.g. domperidone)
- Anti-cholinergic agents (e.g. orphenadrine)
Drowsiness
Tolerance to drowsiness usually develops over 5–7 days, but if troublesome, switching to an alternative opioid often helps. Certain opioids such as fentanyl, morphine and diamorphine (heroin) tend to be particularly sedating, while others such as oxycodone, tilidine and meperidine (pethidine) tend to produce comparatively less sedation, but individual patients responses can vary markedly and some degree of trial and error may be needed to find the most suitable drug for a particular patient. Treatment is at any rate possible—CNS stimulants are generally effective.
- Stimulants (e.g. caffeine, modafinil, amphetamine, methylphenidate)
Itching
Itching tends not to be a severe problem when opioids are used for pain relief, but if required then antihistamines are useful for counteracting itching. Non-sedating antihistamines such as fexofenadine are preferable so as to avoid increasing opioid induced drowsiness, although some sedating antihistamines such as orphenadrine may be helpful as they produce a synergistic analgesic effect which allows smaller doses of opioids to be used while still producing effective analgesia. For this reason some opioid/antihistamine combination products have been marketed, such as Meprozine (meperidine/promethazine) and Diconal (dipipanone/cyclizine), which may also have the added advantage of reducing nausea as well.
- Antihistamines (e.g. fexofenadine)
Constipation
Constipation develops in many people on opioids and since tolerance to this problem does not develop readily, most patients on long-term opioids will need a laxative.[37] Over 30 years experience in palliative care has shown that most opioid constipation can be successfully prevented: "Constipation … is treated [with laxatives and stool-softeners]" (Burton 2004, 277). According to Abse, "It is very important to watch out for constipation, which can be severe" and "can be a very considerable complication" (Abse 1982, 129) if it is ignored. Peripherally acting opioid antagonists such as alvimopan (Entereg) and methylnaltrexone (Relistor) have been found to effectively relieve opioid induced constipation without triggering withdrawal symptoms, although alvimopan is contraindicated in patients who have taken opioids for more than seven days, is only FDA-approved for 15 doses or less, and may increase risk of heart attack.[38][39] For mild cases, a lot of water (around 1.5 L/day) and fiber might suffice (in addition to the laxative and stool-softeners).
- Stool-softening and peristalsis-promoting laxatives (e.g. docusate in combination with bisacodyl or senna).
- Peripherally-acting opioid antagonists (e.g. methylnaltrexone) effectively prevent constipation while not affecting centrally mediated analgesia or provoking withdrawal syndrome, however these can still potentially reduce the efficacy of opioid analgesics in the treatment of conditions where much of the pain relief comes from action at peripherally situated opioid receptors, such as in inflammatory conditions like arthritis or post-surgical pain.
- High water intake and dietary fiber
For more severe and/or chronic cases, the drugs that are used work by not increasing peristalsis, but by preventing water uptake in the intestine, leading to a softer stool with a larger component of water, and, additionally, by acidifying the environment inside the intestine, which both decreases water uptake and enhances peristalsis (e.g. lactulose, which is controversially noted as a possible probiotic). The following drugs are generally efficacious:
- Polyethylene glycol 3350±10% dalton powder for solution (MiraLax, GlycoLax).
- Lactulose syrup
One combination, oxycodone/naloxone, aims to reduce systemic side effects by combining oxycodone with an opioid suppressor, naloxone, in a form which does not pass through the blood–brain barrier. Thus, the constipation effect is suppressed, but not the pain reduction.
Respiratory depression
Respiratory depression is the most serious adverse reaction associated with opioid use, but it usually is seen with the use of a single, intravenous dose in an opioid-naïve patient. In patients taking opioids regularly for pain relief, tolerance to respiratory depression occurs rapidly, so that it is not a clinical problem. Several drugs have been developed which can partially block respiratory depression, although the only respiratory stimulant currently approved for this purpose is doxapram, which has only limited efficacy in this application.[40][41] Newer drugs such as BIMU-8 and CX-546 may however be much more effective.[42][43][44]
- Respiratory stimulants: carotid chemoreceptor agonists (e.g. doxapram), 5-HT4 agonists (e.g. BIMU8), δ-opioid agonists (e.g. BW373U86) and AMPAkines (e.g. CX717) can all reduce respiratory depression caused by opioids without affecting analgesia, but most of these drugs are only moderately effective or have side effects which preclude use in humans. 5-HT1A agonists such as 8-OH-DPAT and repinotan also counteract opioid-induced respiratory depression, but at the same time reduce analgesia, which limits their usefulness for this application.
- Opioid antagonists (e.g. naloxone, nalmefene, diprenorphine)
Hyperalgesia
Opioid-induced hyperalgesia has been observed in some patients, whereby individuals using opioids to relieve pain may paradoxically experience more pain as a result of their medication. This phenomenon, although uncommon, is seen in some palliative care patients, most often when dose is escalated rapidly.[45][46] If encountered, rotation between several different opioid analgesics may mitigate the development of hyperalgesia.[47][48]
Side effects such as hyperalgesia and allodynia, sometimes accompanied by a worsening of neuropathic pain, may be consequences of long-term treatment with opioid analgesics, especially when increasing tolerance has resulted in loss of efficacy and consequent progressive dose escalation over time. This appears to largely be a result of actions of opioid drugs at targets other than the three classic opioid receptors, including the nociceptin receptor, sigma receptor and Toll-like receptor 4, and can be counteracted in animal models by antagonists at these targets such as J-113,397, BD-1047 or (+)-Naloxone respectively.[49][50][51][52] No drugs are currently approved specifically for counteracting opioid-induced hyperalgesia in humans and in severe cases the only solution may be to discontinue use of opioid analgesics and replace them with non-opioid analgesic drugs. However since individual sensitivity to the development of this side effect is highly dose dependent and may vary depending which opioid analgesic is used, many patients can avoid this side effect simply through dose reduction of the opioid drug (usually accompanied by addition of a supplemental non-opioid analgesic), rotating between different opioid drugs, or by switching to a milder opioid with mixed mode of action that also counteracts neuropathic pain, particularly tramadol or tapentadol.[53][54][55][56]
- NMDA antagonists such as ketamine
- SNRIs such as milnacipran
- anticonvulsants such as gabapentin or pregabalin
Increased mortality
Oxycodone and codeine may double mortality as compared to hydrocodone.[57] In contrast to hydrocodone, codeine is metabolized by cytochrome P-450 CYP2D6, which may lead to variable pharmacokinetics due to single-nucleotide polymorphisms and drug interactions. Although oxycodone is metabolized by CYP2D6, it only accounts for a minor portion, whereas CYP3A4 plays a greater role; thus clinically oxycodone metabolism is rarely affected by variants in single-nucleotide polymorphisms.[58]
Hormone imbalance
Clinical studies have consistently associated medical and recreational opioid use with hypogonadism and hormone imbalance in both genders. The effect is dose–dependent. Most studies suggest that the majority (perhaps as much as 90%) of chronic opioid users suffer hormone imbalances. Opioids can also interfere with menstruation in women by limiting the production of luteinizing hormone (LH). Opioid-induced endocrinopathy likely causes the strong association of opioid use with osteoporosis and bone fracture. It also may increase pain and thereby interfere with the intended clinical effect of opioid treatment. Opioid-induced endocrinopathy is likely caused their agonism of opioid receptors in the hypothalamus and the pituitary gland. A study conducted on heroin addicts found that their testosterone levels returned to normal within one month of abstinence, suggesting that the effect is temporary. As of 2013, the effect of low-dose or acute opiate use on the endocrine system is unclear.[59][60]
Disruption of work
Use of opioids may be a risk factor for failing to return to work.[61][62]
In addition, lack of employment may be a predictor of aberrant use of prescription opioids.[63]
People who take opioids long term have increased likelihood of being unemployed.[64] Taking opioids further disrupts the patient's life and the adverse effects of opioids themselves can become a significant barrier to patients having an active life, gaining employment, and sustaining a career.[3]
Increased accident-proneness
Opioid use may increase accident-proneness. Opioids may increase risk of traffic accidents[65][66] and accidental falls.[67]
Rare side effects
Infrequent adverse reactions in patients taking opioids for pain relief include: dose-related respiratory depression (especially with more potent opioids), confusion, hallucinations, delirium, urticaria, hypothermia, bradycardia/tachycardia, orthostatic hypotension, dizziness, headache, urinary retention, ureteric or biliary spasm, muscle rigidity, myoclonus (with high doses), and flushing (due to histamine release, except fentanyl and remifentanil).[11] Both therapeutic and chronic use of opioids can compromise the function of the immune system. Opioids decrease the proliferation of macrophage progenitor cells and lymphocytes, and affect cell differentiation (Roy & Loh, 1996). Opioids may also inhibit leukocyte migration. However the relevance of this in the context of pain relief is not known.
Interactions
The concurrent use of opioids with benzodiazepines or alcohol increases the rates of adverse events, overdose, and death in patients.[3][68] These risks are lessened with close monitoring by a physician, who may conducting ongoing screening for changes in patient behavior and treatment compliance.[68] Physicians treating patients using opioids in combination with other drugs keep continual documentation that further treatment is indicated and remain aware of opportunities to adjust treatment if the patient's condition changes to merit less risky therapy.[68] When opioids are combined with sedatives then the patient has increased risk of breathing problems.[69]
Opioid antagonist
Finally, opioid effects (adverse or otherwise) can be reversed with an opioid antagonist such as naloxone or naltrexone.[70] These competitive antagonists bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This displaces the agonist, attenuating and/or reversing the agonist effects. However, the elimination half-life of naloxone can be shorter than that of the opioid itself, so repeat dosing or continuous infusion may be required, or a longer acting antagonist such as nalmefene may be used. In patients taking opioids regularly it is essential that the opioid is only partially reversed to avoid a severe and distressing reaction of waking in excruciating pain. This is achieved by not giving a full dose but giving this in small doses until the respiratory rate has improved. An infusion is then started to keep the reversal at that level, while maintaining pain relief. Opioid antagonists remain the standard treatment for respiratory depression following opioid overdose, with naloxone being by far the most commonly used, although the longer acting antagonist nalmefene may be used for treating overdoses of long-acting opioids such as methadone, and diprenorphine is used for reversing the effects of extremely potent opioids used in veterinary medicine such as etorphine and carfentanil. However since opioid antagonists also block the beneficial effects of opioid analgesics, they are generally useful only for treating overdose, with use of opioid antagonists alongside opioid analgesics to reduce side effects, requiring careful dose titration and often being poorly effective at doses low enough to allow analgesia to be maintained.
Pharmacology
| Drug | Relative Potency[71] | Nonionized Fraction | Protein Binding | Lipid Solubility[72] |
|---|---|---|---|---|
| Morphine | 1 | ++ | ++ | ++ |
| Meperidine | 0.1 | + | +++ | ++ |
| Hydromorphone | 10 | |||
| Alfentanil | 10–25 | ++++ | ++++ | +++ |
| Fentanyl | 75–125 | + | +++ | ++++ |
| Remifentanil | 250 | +++ | +++ | ++ |
| Sufentanil | 500–1000 | ++ | ++++ | ++++ |
| Etorphine | 1000–3000 |
Opioids bind to specific opioid receptors in the nervous system and other tissues. There are three principal classes of opioid receptors, μ, κ, δ (mu, kappa, and delta), although up to seventeen have been reported, and include the ε, ι, λ, and ζ (Epsilon, Iota, Lambda and Zeta) receptors. Conversely, σ (Sigma) receptors are no longer considered to be opioid receptors because their activation is not reversed by the opioid inverse-agonist naloxone, they do not exhibit high-affinity binding for classical opioids, and they are stereoselective for dextro-rotatory isomers while the other opioid receptors are stereo-selective for laevo-rotatory isomers. In addition, there are three subtypes of μ-receptor: μ1 and μ2, and the newly discovered μ3. Another receptor of clinical importance is the opioid-receptor-like receptor 1 (ORL1), which is involved in pain responses as well as having a major role in the development of tolerance to μ-opioid agonists used as analgesics. These are all G-protein coupled receptors acting on GABAergic neurotransmission.
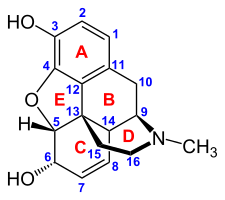
The pharmacodynamic response to an opioid depends upon the receptor to which it binds, its affinity for that receptor, and whether the opioid is an agonist or an antagonist. For example, the supraspinal analgesic properties of the opioid agonist morphine are mediated by activation of the μ1 receptor; respiratory depression and physical dependence by the μ2 receptor; and sedation and spinal analgesia by the κ receptor[citation needed]. Each group of opioid receptors elicits a distinct set of neurological responses, with the receptor subtypes (such as μ1 and μ2 for example) providing even more [measurably] specific responses. Unique to each opioid is its distinct binding affinity to the various classes of opioid receptors (e.g. the μ, κ, and δ opioid receptors are activated at different magnitudes according to the specific receptor binding affinities of the opioid). For example, the opiate alkaloid morphine exhibits high-affinity binding to the μ-opioid receptor, while ketazocine exhibits high affinity to ĸ receptors. It is this combinatorial mechanism that allows for such a wide class of opioids and molecular designs to exist, each with its own unique effect profile. Their individual molecular structure is also responsible for their different duration of action, whereby metabolic breakdown (such as N-dealkylation) is responsible for opioid metabolism.
Functional selectivity
A new strategy of drug development takes receptor signal transduction into consideration. The strategy strives to increase activation of desirable, and to reduce impact on undesirable, signalling pathways. This differential strategy has been given several names, including functional selectivity and biased agonism. The first opioid that was intentionally designed as a biased agonist and placed into clinical evaluation is a chemical compound with the code number TRV130. It displays analgesic activity and reduced adverse effects.[73]
Opioid comparison
Extensive research has been conducted to determine equivalence ratios comparing the relative potency of opioids. Given a dose of an opioid, an equianalgesic chart is used to find the equivalent dosage of another.
History
Non-clinical use was criminalized in the U.S by the Harrison Narcotics Tax Act of 1914, and by other laws worldwide. Since then, nearly all non-clinical use of opioids has been rated zero on the scale of approval of nearly every social institution. However, in United Kingdom the 1926 report of the Departmental Committee on Morphine and Heroin Addiction under the Chairmanship of the President of the Royal College of Physicians reasserted medical control and established the "British system" of control—which lasted until the 1960s; in the U.S. the Controlled Substances Act of 1970 markedly relaxed the harshness of the Harrison Act.
Before the twentieth century, institutional approval was often higher, even in Europe and America. In some cultures, approval of opioids was significantly higher than approval of alcohol. Opiates were used to treat depression and anxiety until the mid-1950s.[74]
Society and culture
Global shortages
Morphine and other poppy-based medicines have been identified by the World Health Organization as essential in the treatment of severe pain. However, only six countries use 77% of the world's morphine supplies, leaving many emerging countries lacking in pain relief medication.[75] The current system of supply of raw poppy materials to make poppy-based medicines is regulated by the International Narcotics Control Board under the provision of the 1961 Single Convention on Narcotic Drugs. The amount of raw poppy materials that each country can demand annually based on these provisions must correspond to an estimate of the country's needs taken from the national consumption within the preceding two years. In many countries, underprescription of morphine is rampant because of the high prices and the lack of training in the prescription of poppy-based drugs. The World Health Organization is now working with administrations from various countries to train healthworkers and to develop national regulations regarding drug prescription to facilitate a greater prescription of poppy-based medicines.[76]
Another idea to increase morphine availability is proposed by the Senlis Council, who suggest, through their proposal for Afghan Morphine, that Afghanistan could provide cheap pain relief solutions to emerging countries as part of a second-tier system of supply that would complement the current INCB regulated system by maintaining the balance and closed system that it establishes while providing finished product morphine to those suffering from severe pain and unable to access poppy-based drugs under the current system.
United States approval
The sole clinical indications for opioids in the United States, according to Drug Facts and Comparisons, 2005, are:
- Moderate to severe pain, i.e., to provide analgesia or, in surgery, to induce and maintain anesthesia, as well as allaying patient apprehension right before the procedure. Fentanyl, oxymorphone, hydromorphone, and morphine are most commonly used for this purpose, in conjunction with other drugs such as scopolamine, short and intermediate-acting barbiturates, and benzodiazepines, especially midazolam which has a rapid onset of action and shorter duration than diazepam (Valium) or similar drugs. The enhancement of the effects of each drug by the others is useful in troublesome procedures like endoscopies, complicated and difficult deliveries (pethidine and its relatives and piritramide where it is used are favoured by many practitioners with morphine and derivatives as the second line), incision & drainage of severe abscesses, intraspinal injections, and minor and moderate-impact surgical procedures in patients unable to have general anesthesia due to allergy to some of the drugs involved or other concerns.
- Cough (codeine, dihydrocodeine, ethylmorphine (dionine), hydromorphone and hydrocodone, with morphine or methadone as a last resort.)
- Diarrhea (generally loperamide, difenoxin or diphenoxylate; but paregoric, powdered opium or laudanum or morphine may be used in some cases of severe diarrheal diseases, e.g. cholera); also diarrhea secondary to Irritable Bowel Syndrome (Codeine, paregoric, diphenoxylate, difenoxin, loperamide, laudanum)
- Anxiety due to shortness of breath (oxymorphone and dihydrocodeine only)
- Opioid dependence (methadone and buprenorphine only)
Evidence supports the use of low dose, regular oral opioids for the safe relief of breathlessness that is not responsive to disease-modifying treatments. This action appears to be a result of the effect on opioid receptors in the limbic system.
Opioids are not used for psychological relief.
Opioids are often used in combination with adjuvant analgesics (drugs which have an indirect effect on the pain). In palliative care, opioids are not recommended for sedation or anxiety because experience has found them to be ineffective agents in these roles. Some opioids are relatively contraindicated in renal failure because of the accumulation of the parent drug or their active metabolites (e.g. codeine and oxycodone). Age (young or old) is not a contraindication to strong opioids. Some synthetic opioids such as pethidine have metabolites which are actually neurotoxic and should therefore be used only in acute situations.
Recreational use
Drug misuse is the use of drugs for reasons other than what the drug was prescribed for. Opioids are primarily misused due to their ability to produce euphoria. Misuse can also include giving drugs to people for whom it was not prescribed or selling the medication, both of which are crimes punishable by imprisonment in some, if not most, countries.
Classification
There are a number of broad classes of opioids:
- Natural opiates: alkaloids contained in the resin of the opium poppy, primarily morphine, codeine, and thebaine, but not papaverine and noscapine which have a different mechanism of action; The following could be considered natural opiates: The leaves from Mitragyna speciosa (also known as kratom) contain a few naturally-occurring opioids, active via Mu- and Delta receptors. Salvinorin A, found naturally in the Salvia divinorum plant, is a kappa-opioid receptor agonist.
- Esters of morphine opiates: slightly chemically altered but more natural than the semi-synthetics, as most are morphine prodrugs, diacetylmorphine (morphine diacetate; heroin), nicomorphine (morphine dinicotinate), dipropanoylmorphine (morphine dipropionate), desomorphine, acetylpropionylmorphine, dibenzoylmorphine, diacetyldihydromorphine;[77]
- Semi-synthetic opioids: created from either the natural opiates or morphine esters, such as hydromorphone, hydrocodone, oxycodone, oxymorphone, ethylmorphine and buprenorphine;
- Fully synthetic opioids: such as fentanyl, pethidine, levorphanol, methadone, tramadol and dextropropoxyphene;
- Endogenous opioid peptides, produced naturally in the body, such as endorphins, enkephalins, dynorphins, and endomorphins. Morphine, and some other opioids, which are produced in small amounts in the body, are included in this category.
- There are also drugs such as tramadol and tapentadol that are chemically not of the opioid class, but do have agonist actions at the μ-opioid receptor. Although their exact mechanism of action is not fully understood, they both have a dual mode of action, the second mode of action appearing to be on the noradrenergic and serotonergic systems.[78] This second mechanism of action was discovered during testing in where the drugs showed signs of analgesia even when naloxone, an opioid antagonist, was administered.[79]
Some minor opium alkaloids and various substances with opioid action are also found elsewhere, including molecules present in kratom, Corydalis, and Salvia divinorum plants and some species of poppy aside from Papaver somniferum. There are also strains which produce copious amounts of thebaine, an important raw material for making many semi-synthetic and synthetic opioids. Of all of the more than 120 poppy species, only two produce morphine.
Amongst analgesics are a small number of agents which act on the central nervous system but not on the opioid receptor system and therefore have none of the other (narcotic) qualities of opioids although they may produce euphoria by relieving pain—a euphoria that, because of the way it is produced, does not form the basis of habituation, physical dependence, or addiction. Foremost amongst these are nefopam, orphenadrine, and perhaps phenyltoloxamine and/or some other antihistamines. Tricyclic antidepressants have painkilling effect as well, but they're thought to do so by indirectly activating the endogenous opioid system. Paracetamol is predominantly a centrally acting analgesic (non-narcotic) which mediates its effect by action on descending serotoninergic (5-hydroxy triptaminergic) pathways, to increase 5-HT release (which inhibits release of pain mediators). It also decreases cyclo-oxygenase activity. It has recently been discovered that most or all of the therapeutic efficacy of paracetamol is due to a metabolite ( AM404, making paracetamol a prodrug) which enhances the release of serotonin and also interacts as with the cannabinoid receptors by inhibiting the uptake of anandamide.[citation needed]
Other analgesics work peripherally (i.e., not on the brain or spinal cord). Research is starting to show that morphine and related drugs may indeed have peripheral effects as well, such as morphine gel working on burns. Recent investigations discovered opioid receptors on peripheral sensory neurons.[80] A significant fraction (up to 60%) of opioid analgesia can be mediated by such peripheral opioid receptors, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain.[81] Inflammatory pain is also blunted by endogenous opioid peptides activating peripheral opioid receptors.[82]
It has been discovered in 1953,[citation needed] that the human body, as well as those of some other animals, naturally produce minute amounts of morphine and codeine and possibly some of their simpler derivatives like heroin and dihydromorphine, in addition to the well known endogenous opioid peptides. Some bacteria are capable of producing some semi-synthetic opioids such as hydromorphone and hydrocodone when living in a solution containing morphine or codeine respectively.
Many of the alkaloids and other derivatives of the opium poppy are not opioids or narcotics; the best example is the smooth-muscle relaxant papaverine. Noscapine is a marginal case as it does have CNS effects but not necessarily similar to morphine, and it is probably in a category all its own.
Dextromethorphan (the stereoisomer of levomethorphan, a semi-synthetic opioid agonist) and its metabolite dextrorphan have no opioid analgesic effect at all despite their structural similarity to other opioids; instead they are potent NMDA antagonists and sigma 1 and 2-receptor agonists and are used in many over-the-counter cough suppressants.
Salvinorin A is a unique selective, powerful ĸ-opioid receptor agonist. It is not properly considered an opioid nevertheless, because:
- chemically, it is not an alkaloid; and
- it has no typical opioid properties: absolutely no anxiolytic or cough-suppressant effects. It is instead a powerful hallucinogen.
| Opioid peptides | Skeletal molecular images |
|---|---|
| Adrenorphin |  |
| Amidorphin |  |
| Casomorphin |  |
| DADLE | |
| DAMGO |  |
| Dermorphin | |
| Endomorphin |  |
| Morphiceptin |  |
| Nociceptin |  |
| Octreotide |  |
| Opiorphin |  |
| TRIMU 5 |  |
Endogenous opioids
Opioid-peptides that are produced in the body include:
- Endorphins
- Enkephalins
- Dynorphins
- Endomorphins
β-endorphin is expressed in Pro-opiomelanocortin (POMC) cells in the arcuate nucleus, in the brainstem and in immune cells, and acts through μ-opioid receptors. β-endorphin has many effects, including on sexual behavior and appetite. β-endorphin is also secreted into the circulation from pituitary corticotropes and melanotropes. α-neo-endorphin is also expressed in POMC cells in the arcuate nucleus.
met-enkephalin is widely distributed in the CNS and in immune cells; [met]-enkephalin is a product of the proenkephalin gene, and acts through μ and δ-opioid receptors. leu-enkephalin, also a product of the proenkephalin gene, acts through δ-opioid receptors.
Dynorphin acts through κ-opioid receptors, and is widely distributed in the CNS, including in the spinal cord and hypothalamus, including in particular the arcuate nucleus and in both oxytocin and vasopressin neurons in the supraoptic nucleus.
Endomorphin acts through μ-opioid receptors, and is more potent than other endogenous opioids at these receptors.
Opium alkaloids
Phenanthrenes naturally occurring in (opium):
Preparations of mixed opium alkaloids, including papaveretum, are still occasionally used.
Esters of morphine
- Diacetylmorphine (morphine diacetate; heroin)
- Nicomorphine (morphine dinicotinate)
- Dipropanoylmorphine (morphine dipropionate)
- Diacetyldihydromorphine
- Acetylpropionylmorphine
- Desomorphine
- Methyldesorphine
- Dibenzoylmorphine
Ethers of morphine
Semi-synthetic alkaloid derivatives
Synthetic opioids
Anilidopiperidines
- Fentanyl
- Alphamethylfentanyl
- Alfentanil
- Sufentanil
- Remifentanil
- Carfentanyl
- Ohmefentanyl
Phenylpiperidines
- Pethidine (meperidine)
- Ketobemidone
- MPPP
- Allylprodine
- Prodine
- PEPAP
Diphenylpropylamine derivatives
- Propoxyphene
- Dextropropoxyphene
- Dextromoramide
- Bezitramide
- Piritramide
- Methadone
- Dipipanone
- Levomethadyl Acetate (LAAM)
- Difenoxin
- Diphenoxylate
- Loperamide (does cross the blood-brain barrier but is quickly pumped into the non-central nervous system by P-Glycoprotein. Mild opiate withdrawal in animal models exhibits this action after sustained and prolonged use including rhesus monkeys, mice, and rats.)
Benzomorphan derivatives
- Dezocine—agonist/antagonist
- Pentazocine—agonist/antagonist
- Phenazocine
Oripavine derivatives
- Buprenorphine—partial agonist
- Dihydroetorphine
- Etorphine
Morphinan derivatives
- Butorphanol—agonist/antagonist
- Nalbuphine—agonist/antagonist
- Levorphanol
- Levomethorphan
Others
- Lefetamine
- Menthol (Kappa-Opioid agonist)
- Meptazinol
- Mitragynine
- Tilidine
- Tramadol
- Tapentadol
Allosteric modulators
Opioid antagonists
Table of morphinan opioids
| Table of morphinan opioids: click to | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table of non-morphinan opioids
| Table of non-morphinan opioids: click to | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
- Froehde reagent
- Opiate comparison
References
- ↑ Alexander GC, Kruszewski SP, Webster DW (2012). "Rethinking Opioid Prescribing to Protect Patient Safety and Public Health". JAMA 308 (18): 1865–1866. doi:10.1001/jama.2012.14282. PMID 23150006.
- ↑ 2.0 2.1 Okie S (November 2010). "A flood of opioids, a rising tide of deaths". N. Engl. J. Med. 363 (21): 1981–5. doi:10.1056/NEJMp1011512. PMID 21083382.
Responses to Okie's perspective: "Opioids and deaths". N. Engl. J. Med. 364 (7): 686–7. February 2011. doi:10.1056/NEJMc1014490. - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 Consumer Reports Health Best Buy Drugs (21 August 2012), "Using Opioids to Treat: Chronic Pain - Comparing Effectiveness, Safety, and Price", Opioids, Yonkers, New York: Consumer Reports, retrieved 28 October 2013
- ↑ McNicol, E. D.; Strassels, S.; Goudas, L.; Lau, J.; Carr, D. B. (2005). "NSAIDS or paracetamol, alone or combined with opioids, for cancer pain". In McNicol, Ewan D. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD005180.
- ↑ 5.0 5.1 Regarding using opioids to treat migraine, see American Academy of Neurology (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (American Academy of Neurology), retrieved August 1, 2013, which cites
- Silberstein, S. D. (2000). "Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology 55 (6): 754–762. doi:10.1212/WNL.55.6.754. PMID 10993991.
- Evers, S.; Afra, J.; Frese, A.; Goadsby, P. J.; Linde, M.; May, A.; Sándor, P. S.; European Federation of Neurological Societies (2009). "EFNS guideline on the drug treatment of migraine - revised report of an EFNS task force". European Journal of Neurology 16 (9): 968–981. doi:10.1111/j.1468-1331.2009.02748.x. PMID 19708964.
- Institute for Clinical Systems Improvement (2011), Headache, Diagnosis and Treatment of, Institute for Clinical Systems Improvement
- ↑ Painter, J. T.; Crofford, L. J. (2013). "Chronic Opioid Use in Fibromyalgia Syndrome". Journal of Clinical Rheumatology 19 (2): 72–77. doi:10.1097/RHU.0b013e3182863447. PMID 23364665.
- ↑ McNicol, E. D.; Midbari, A.; Eisenberg, E. (2013). "Opioids for neuropathic pain". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD006146.pub2.
- ↑ "nalbuphine - injection, Nubain". MedicineNet.com. Retrieved 31 January 2014.
- ↑ 9.0 9.1 9.2 American Headache Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (American Headache Society), retrieved 10 December 2013, which cites
- Bigal, M. E.; Lipton, R. B. (2009). "Excessive opioid use and the development of chronic migraine". Pain 142 (3): 179–182. doi:10.1016/j.pain.2009.01.013. PMID 19232469.
- Bigal, M. E.; Serrano, D.; Buse, D.; Scher, A.; Stewart, W. F.; Lipton, R. B. (2008). "Acute Migraine Medications and Evolution from Episodic to Chronic Migraine: A Longitudinal Population-Based Study". Headache: the Journal of Head and Face Pain 48 (8): 1157–1168. doi:10.1111/j.1526-4610.2008.01217.x. PMID 18808500.
- Scher, A. I.; Stewart, W. F.; Ricci, J. A.; Lipton, R. B. (2003). "Factors associated with the onset and remission of chronic daily headache in a population-based study". Pain 106 (1–2): 81–89. doi:10.1016/S0304-3959(03)00293-8. PMID 14581114.
- Katsarava, Z.; Schneeweiss, S.; Kurth, T.; Kroener, U.; Fritsche, G.; Eikermann, A.; Diener, H. C.; Limmroth, V. (2004). "Incidence and predictors for chronicity of headache in patients with episodic migraine". Neurology 62 (5): 788–790. doi:10.1212/01.WNL.0000113747.18760.D2. PMID 15007133.
- ↑ 10.0 10.1 10.2 10.3 Noble, M.; Treadwell, J. R.; Tregear, S. J.; Coates, V. H.; Wiffen, P. J.; Akafomo, C.; Schoelles, K. M. (2010). "Long-term opioid management for chronic noncancer pain". In Noble, Meredith. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD006605.pub2.
- ↑ 11.0 11.1 11.2 11.3 11.4 Doyle, D.; Hanks, G.; Cherney, I. et al., eds. (2004). Oxford Textbook of Palliative Medicine (3rd ed.). Oxford University Press. ISBN 0198566980.
- ↑ Schneider JP. Rational use of opioid analgesics in chronic musculoskeletal pain. J Musculoskel Med. 2010;27:142-148.
- ↑ Baumann S (2009). "A nursing approach to pain in older adults". Medsurg Nurs 18 (2): 77–82; quiz 83. PMID 19489204.
- ↑ Buckeridge D, Huang A, Hanley J, Kelome A, Reidel K, Verma A, Winslade N, Tamblyn R (September 2010). "Risk of injury associated with opioid use in older adults". J Am Geriatr Soc 58 (9): 1664–70. doi:10.1111/j.1532-5415.2010.03015.x. PMID 20863326.
- ↑ 15.0 15.1 Wolff, K (2002). "Characterization of methadone overdose: Clinical considerations and the scientific evidence". Therapeutic drug monitoring 24 (4): 457–70. PMID 12142628.
- ↑ Teichtahl, H; Wang, D (2007). "Sleep-disordered breathing with chronic opioid use". Expert Opinion on Drug Safety 6 (6): 641–9. doi:10.1517/14740338.6.6.641. PMID 17967153.
- ↑ Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M (January 2010). "Opioid prescriptions for chronic pain and overdose: a cohort study". Ann Intern Med 152 (2): 85–92. doi:10.1059/0003-4819-152-2-201001190-00006. PMC 3000551. PMID 20083827.
- ↑ Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN (April 2011). "Opioid dose and drug-related mortality in patients with nonmalignant pain". Arch Intern Med 171 (7): 686–91. doi:10.1001/archinternmed.2011.117. PMID 21482846.
- ↑ Pradhan AA 'et al. (2010): J Neurosci, 30(49):16459-68. PMID 21147985
- ↑ Kollars JP, Larson MD (March 2005). "Tolerance to miotic effects of opioids". Anesthesiology 102 (3): 701. doi:10.1097/00000542-200503000-00047. PMID 15731628.
- ↑ Santillán R, Maestre JM, Hurlé MA, Flórez J (Jul 1994). "Enhancement of opiate analgesia by nimodipine in cancer patients chronically treated with morphine: a preliminary report". Pain 58 (1): 129–32. doi:10.1016/0304-3959(94)90192-9. PMID 7970835.
- ↑ Smith FL, Dombrowski DS, Dewey WL (Feb 1999). "Involvement of intracellular calcium in morphine tolerance in mice". Pharmacology, Biochemistry, and Behavior 62 (2): 381–8. doi:10.1016/S0091-3057(98)00168-3. PMID 9972707.
- ↑ McCarthy RJ, Kroin JS, Tuman KJ, Penn RD, Ivankovich AD (Apr 1998). "Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats". Anesthesia and Analgesia 86 (4): 830–6. PMID 9539610.
- ↑ Larson AA, Kovács KJ, Spartz AK (Nov 2000). "Intrathecal Zn2+ attenuates morphine antinociception and the development of acute tolerance". European Journal of Pharmacology 407 (3): 267–72. doi:10.1016/S0014-2999(00)00715-9. PMID 11068022.
- ↑ Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS (February 1996). "Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors". Eur. J. Pharmacol. 297 (1–2): 27–33. doi:10.1016/0014-2999(95)00728-8. PMID 8851162.
- ↑ Malec, D; Malec D, Mandryk M, Fidecka S (Mar–Apr 2008). "Interaction of memantine and ketamine in morphine- and pentazocine-induced antinociception in mice". Pharmacological Reports 60 (2): 149–55. PMID 18443375. Retrieved September 17, 2011.
- ↑ McCleane GJ (2003). "The cholecystokinin antagonist proglumide enhances the analgesic effect of dihydrocodeine". Clin J Pain 19 (3): 200–1. doi:10.1097/00002508-200305000-00008. PMID 12792559.
- ↑ Watkins LR, Kinscheck IB, Mayer DJ (Apr 1984). "Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide". Science 224 (4647): 395–6. doi:10.1126/science.6546809. PMID 6546809.
- ↑ Tang J, Chou J, Iadarola M, Yang HY, Costa E (Jun 1984). "Proglumide prevents and curtails acute tolerance to morphine in rats". Neuropharmacology 23 (6): 715–8. doi:10.1016/0028-3908(84)90171-0. PMID 6462377.
- ↑ Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW (Jul 2007). "Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes". Expert Opinion on Investigational Drugs 16 (7): 935–50. doi:10.1517/13543784.16.7.935. PMID 17594181.
- ↑ Hermann D, Klages E, Welzel H, Mann K, Croissant B (June 2005). "Low efficacy of non-opioid drugs in opioid withdrawal symptoms". Addict Biol 10 (2): 165–9. doi:10.1080/13556210500123514. PMID 16191669.
- ↑ Bannwarth, B. (Sep 10, 2012). "Will abuse deterrent formulations of opioid analgesics be successful in their purpose?". Drugs 72 (12): 1713–1723. doi:10.2165/11635860-000000000-00000. PMID 22931520.
- ↑ Schneider, J.P.; Matthews, M., Jamison, R.N. (24 Oct 2010). "Abuse-deterrent and tamper-resistant opioid formulations: what is their role in addressing prescription opioid abuse?". CNS Drugs 10 (80): 805–810. doi:10.2165/11584260-000000000-00000. PMID 20839893.
- ↑ 34.0 34.1 34.2 34.3 34.4 34.5 34.6 34.7 Furlan, A. D.; Sandoval, J. A.; Mailis-Gagnon, A.; Tunks, E. (2006). "Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects". Canadian Medical Association Journal 174 (11): 1589–1594. doi:10.1503/cmaj.051528. PMC 1459894. PMID 16717269.
- ↑ Xu, Y.; Johnson, A. (2013). "Opioid Therapy Pharmacogenomics for Noncancer Pain: Efficacy, Adverse Events, and Costs". Pain Research and Treatment 2013: 1. doi:10.1155/2013/943014. PMC 3791560. PMID 24167729.
- ↑ Brush, D. E. (2012). "Complications of Long-Term Opioid Therapy for Management of Chronic Pain: The Paradox of Opioid-Induced Hyperalgesia". Journal of Medical Toxicology 8 (4): 387–392. doi:10.1007/s13181-012-0260-0. PMC 3550256. PMID 22983894.
- ↑ McCarberg, B. (2013). "Overview and Treatment of Opioid-Induced Constipation". Postgraduate Medicine 125 (4): 7–17. doi:10.3810/pgm.2013.07.2651. PMID 23782897.
- ↑ McNicol ED, Boyce D, Schumann R, Carr DB (2008). "Mu-opioid antagonists for opioid-induced bowel dysfunction". In McNicol, Ewan D. Cochrane Database Syst Rev (2): CD006332. doi:10.1002/14651858.CD006332.pub2. PMID 18425947.
- ↑ Portenoy RK, Thomas J, Moehl Boatwright ML et al. (May 2008). "Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study". J Pain Symptom Manage 35 (5): 458–68. doi:10.1016/j.jpainsymman.2007.12.005. PMID 18440447.
- ↑ Yost CS (2006). "A new look at the respiratory stimulant doxapram". CNS Drug Rev 12 (3–4): 236–49. doi:10.1111/j.1527-3458.2006.00236.x. PMID 17227289.
- ↑ Tan ZM, Liu JH, Dong T, Li JX (August 2006). "[Clinical observation of target-controlled remifentanil infusion combined with propofol and doxapram in painless artificial abortion]". Nan Fang Yi Ke Da Xue Xue Bao 26 (8): 1206–8. PMID 16939923.
- ↑ Manzke T, Guenther U, Ponimaskin E, Haller M, Dutschmann M, Schwarzacher S, Richter D (2003). "5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia". Science 301 (5630): 226–9. doi:10.1126/science.1084674. PMID 12855812.
- ↑ Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D (August 2007). "5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function". Hypertension 50 (2): 368–76. doi:10.1161/HYPERTENSIONAHA.107.091033. PMID 17576856.
- ↑ Ren J, Poon BY, Tang Y, Funk GD, Greer JJ (December 2006). "Ampakines alleviate respiratory depression in rats". Am. J. Respir. Crit. Care Med. 174 (12): 1384–91. doi:10.1164/rccm.200606-778OC. PMID 16973981.
- ↑ Wilson GR, Reisfield GM (2003). "Morphine hyperalgesia: a case report". Am J Hosp Palliat Care 20 (6): 459–61. doi:10.1177/104990910302000608. PMID 14649563.
- ↑ Vella-Brincat J, Macleod AD (2007). "Adverse effects of opioids on the central nervous systems of palliative care patients". J Pain Palliat Care Pharmacother 21 (1): 15–25. doi:10.1080/J354v21n01_05. PMID 17430825.
- ↑ Mercadante S, Arcuri E (2005). "Hyperalgesia and opioid switching". Am J Hosp Palliat Care 22 (4): 291–4. doi:10.1177/104990910502200411. PMID 16082916.
- ↑ Fine PG (2004). "Opioid insights:opioid-induced hyperalgesia and opioid rotation". J Pain Palliat Care Pharmacother 18 (3): 75–9. doi:10.1080/J354v18n03_08. PMID 15364634.
- ↑ Wu HE, Hong JS, Tseng LF (October 2007). "Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse". European Journal of Pharmacology 571 (2–3): 145–51. doi:10.1016/j.ejphar.2007.06.012. PMC 2080825. PMID 17617400.
- ↑ Díaz JL, Zamanillo D, Corbera J, Baeyens JM, Maldonado R, Pericàs MA, Vela JM, Torrens A (September 2009). "Selective sigma-1 (sigma1) receptor antagonists: emerging target for the treatment of neuropathic pain". Central Nervous System Agents in Medicinal Chemistry 9 (3): 172–83. doi:10.2174/1871524910909030172. PMID 20021351.
- ↑ Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR (January 2010). "Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1β". Neuroscience 165 (2): 569–83. doi:10.1016/j.neuroscience.2009.10.011. PMC 2795035. PMID 19833175.
- ↑ Scoto GM, Aricò G, Iemolo A, Ronsisvalle G, Parenti C (April 2010). "Selective inhibition of the NOP receptor in the ventrolateral periaqueductal gray attenuates the development and the expression of tolerance to morphine-induced antinociception in rats". Peptides 31 (4): 696–700. doi:10.1016/j.peptides.2009.12.028. PMID 20067813.
- ↑ Onal A, Parlar A, Ulker S (December 2007). "Milnacipran attenuates hyperalgesia and potentiates antihyperalgesic effect of tramadol in rats with mononeuropathic pain". Pharmacology, Biochemistry, and Behavior 88 (2): 171–8. doi:10.1016/j.pbb.2007.08.001. PMID 17854875.
- ↑ Mitra S (2008). "Opioid-induced hyperalgesia: pathophysiology and clinical implications". Journal of Opioid Management 4 (3): 123–30. PMID 18717507.
- ↑ Baron R (2009). "Neuropathic pain: a clinical perspective". Handbook of Experimental Pharmacology. Handbook of Experimental Pharmacology 194 (194): 3–30. doi:10.1007/978-3-540-79090-7_1. ISBN 978-3-540-79089-1. PMID 19655103.
- ↑ Candiotti KA, Gitlin MC (July 2010). "Review of the effect of opioid-related side effects on the undertreatment of moderate to severe chronic non-cancer pain: tapentadol, a step toward a solution?". Current Medical Research and Opinion 26 (7): 1677–84. doi:10.1185/03007995.2010.483941. PMID 20465361.
- ↑ Solomon DH, Rassen JA, Glynn RJ, et al. (December 2010). "The comparative safety of opioids for nonmalignant pain in older adults". Arch. Intern. Med. 170 (22): 1979–86. doi:10.1001/archinternmed.2010.450. PMID 21149754.
- ↑ Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC et al. (2010). "Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety". Br J Pharmacol 160 (4): 919–30. doi:10.1111/j.1476-5381.2010.00709.x. PMC 2935998. PMID 20590588.
- ↑ Brennan, M. J. (2013). "The effect of opioid therapy on endocrine function". The American Journal of Medicine 126 (3 Suppl 1): S12–8. doi:10.1016/j.amjmed.2012.12.001. PMID 23414717.
- ↑ Colameco, S; Coren, J. S. (2009). "Opioid-induced endocrinopathy". The Journal of the American Osteopathic Association 109 (1): 20–5. PMID 19193821.
- ↑ Brede E, Mayer TG, Gatchel RJ (2012). "Prediction of failure to retain work 1 year after interdisciplinary functional restoration in occupational injuries". Arch Phys Med Rehabil 93 (2): 268–74. doi:10.1016/j.apmr.2011.08.029. PMID 22289236.
- ↑ Volinn E, Fargo JD, Fine PG (2009). "Opioid therapy for nonspecific low back pain and the outcome of chronic work loss". Pain 142 (3): 194–201. doi:10.1016/j.pain.2008.12.017. PMID 19181448.
- ↑ White KT, Dillingham TR, González-Fernández M, Rothfield L (2009). "Opiates for chronic nonmalignant pain syndromes: can appropriate candidates be identified for outpatient clinic management?". Am J Phys Med Rehabil 88 (12): 995–1001. doi:10.1097/PHM.0b013e3181bc006e. PMID 19789432.
- ↑ Cherubino, P.; Sarzi-Puttini, P.; Zuccaro, S. M.; Labianca, R. (2012). "The Management of Chronic Pain in Important Patient Subgroups". Clinical Drug Investigation 32: 35. doi:10.2165/11630060-000000000-00000.
- ↑ Kaye, A. M.; Kaye, A. D.; Lofton, E. C. (2013). "Basic Concepts in Opioid Prescribing and Current Concepts of Opioid-Mediated Effects on Driving". The Ochsner journal 13 (4): 525–532. PMC 3865831. PMID 24358001.
- ↑ Orriols L, Delorme B, Gadegbeku B, et al. (2010). "Prescription medicines and the risk of road traffic crashes: a French registry-based study". In Pirmohamed, Munir. PLoS Med. 7 (11): e1000366. doi:10.1371/journal.pmed.1000366. PMC 2981588. PMID 21125020.
- ↑ Miller M, Stürmer T, Azrael D, Levin R, Solomon DH (2011). "Opioid analgesics and the risk of fractures in older adults with arthritis". J Am Geriatr Soc 59 (3): 430–8. doi:10.1111/j.1532-5415.2011.03318.x. PMC 3371661. PMID 21391934.
- ↑ 68.0 68.1 68.2 Gudin, J.; Mogali, S.; Jones, J.; Comer, S. (2013). "Risks, Management, and Monitoring of Combination Opioid, Benzodiazepines, and/or Alcohol Use". Postgraduate Medicine 125 (4): 115–130. doi:10.3810/pgm.2013.07.2684. PMID 23933900.
- ↑ Stuth, E. A. E.; Stucke, A. G.; Zuperku, E. J. (2012). Effects of Anesthetics, Sedatives, and Opioids on Ventilatory Control. "Comprehensive Physiology". Comprehensive Physiology 2 (4): 2281–2367. doi:10.1002/cphy.c100061. ISBN 9780470650714. PMID 23720250.
- ↑ Gowing, L.; Ali, R.; White, J. M. (2009). "Opioid antagonists with minimal sedation for opioid withdrawal". In Gowing, Linda. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD002021.pub3.
- ↑ Miller, Ronald D. (2010). Miller's Anesthesia (7th ed.). Elsevier Health Sciences. ISBN 978-0-443-06959-8.
- ↑ Morgan, G. Edward; Mikhail, Maged S.; Murray, Michael J. (2006). Clinical Anesthesiology (4th ed.). McGraw Hill. ISBN 978-0-07-110515-6.
- ↑ DeWire SM et al. (2013): J Pharmacol Exp Ther, 344(3):708-17. PMID 23300227
- ↑ see Abse; Berridge; Bodkin; Callaway; Emrich; Gold; Gutstein; Mongan; Portenoy; Reynolds; Takano; Verebey; Walsh; Way.
- ↑ "Feasibility Study on Opium Licensing in Afghanistan for the Production of Morphine and Other Essential Medicines". ICoS. September 2005.
- ↑ "Assuring Availability of Opioid Analgesics" (PDF). World Health Organization.
- ↑ "Esters of Morphine". United Nations Office on Drugs and Crime. http://www.unodc.org. Retrieved 10 March 2012.
- ↑ "Ultram brand of TRAMADOL HYDROCHLORIDE" www.opiods.com
- ↑ Rojas-Corrales MO, Gibert-Rahola J, Micó JA (1998). "Tramadol induces antidepressant-type effects in mice". Life Sci. 63 (12): PL175–80. doi:10.1016/S0024-3205(98)00369-5. PMID 9749830.
- ↑ Stein C, Schäfer M, Machelska H (2003). "Attacking pain at its source: new perspectives on opioids". Nature Medicine 9 (8): 1003–1008. doi:10.1038/nm908. PMID 12894165.
- ↑ Stein C, Lang LJ (2009). "Peripheral mechanisms of opioid analgesia". Curr Opin Pharmacol 9 (1): 3–8. doi:10.1016/j.coph.2008.12.009. PMID 19157985.
- ↑ Busch-Dienstfertig M, Stein C (2010). "Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain-basic and therapeutic aspects". Brain Behav Immun 24 (5): 683–694. doi:10.1016/j.bbi.2009.10.013. PMID 19879349.
- ↑ Odell LR, Skopec J, McCluskey A (March 2008). "Isolation and identification of unique marker compounds from the Tasmanian poppy Papaver somniferum N. Implications for the identification of illicit heroin of Tasmanian origin". Forensic Sci. Int. 175 (2–3): 202–8. doi:10.1016/j.forsciint.2007.07.002. PMID 17765420.
- ↑ 84.0 84.1 Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (February 2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn Schmiedebergs Arch. Pharmacol. 372 (5): 354–61. doi:10.1007/s00210-006-0033-x. PMID 16489449.
External links
- Opioid Withdrawal Symptoms—Information about Opioid and opiate withdrawal issues
- World Health Organization guidelines for the availability and accessibility of controlled substances
- Reference list to the previous publication
- Links to all language versions of the previous publication
- Video: Opioid side effects (Vimeo) (YouTube)—A short educational film about the practical management of opioid side effects.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||













































































































.png)










-Tramadol.svg.png)

piperidylidene-2-(4-chlorophenyl)sulfonamide.png)