Ombuin
From Wikipedia, the free encyclopedia
| Ombuin | ||
|---|---|---|
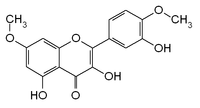 | ||
| IUPAC name 3,5-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-methoxychromen-4-one | ||
| Other names 4',7-Dimethylquercetin | ||
| Identifiers | ||
| CAS number | 529-40-8 | |
| PubChem | 5320287 | |
| ChEBI | CHEBI:67493 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C17H14O7 | |
| Molar mass | 330.29 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Ombuin is an O-methylated flavonol, a type of flavonoid. It is the 4',7-O-methyl derivative of quercetin.
Ombuin can be found in species of the genus Erythroxylum. It can also be synthetized.[1] Ombuin 3-sulfate can be isolated from Flaveria chloraefolia.[2]
Glycosides
Ombuin-3-rutinoside can be isolated from Phytolacca dioica, the ombu tree.[3] Ombuin-3-O-rhamnosylglucoside can be found in Erythroxylum rufum.[4]
Other glycosides (ombuosides) :
- Ombuin 3-galactoside (C23H24O12, CAS number 69168-13-4)
- Ombuin 3-glucoside (C23H24O12, CAS number 158642-42-3)
References
- ↑ Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin. Koppaka V. Rao, Jacob A. Owoyale, Journal of Heterocyclic Chemistry, Volume 13 Issue 6, Pages 1293 - 1295
- ↑ Ombuin 3-sulphate from Flaveria chloraefolia. Denis Barron and Ragai K. Ibrahim, Phytochemistry, Volume 27, Issue 7, 1988, pages 2362-2363, doi:10.1016/0031-9422(88)80166-3
- ↑ Über die Synthese von Quercetin-3-glykosiden, II. Synthese des Ombuosids und eine rationelle Synthese von Rutin. Ludwig Hörhammer, Hildebert Wagner, Hans-Günther Arndt, Gustav Hitzler, Lorand Farkas, Chemische Berichte, Volume 101 Issue 4, Pp 1183-1185, 1968 (German)
- ↑ Flavonoids of Erythroxylum rufum and Erythroxylum ulei. Bruce A. Bohm, David W. Phillips and Fred R. Gander, J. Nat. Prod., 1981, volume 44, issue 6, pages 676–679, doi:10.1021/np50018a009
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.