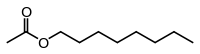
Octyl acetate
From Wikipedia, the free encyclopedia
| Octyl acetate | |
|---|---|
 | |
| IUPAC name Octyl acetate | |
| Other names Octyl ethanoate | |
| Identifiers | |
| CAS number | 112-14-1 |
| PubChem | 8164 |
| ChemSpider | 7872 |
| RTECS number | AJ1400000 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C10H20O2 |
| Molar mass | 172.26 g mol−1 |
| Appearance | Colorless liquid |
| Density | 0.87 g/cm³[1] |
| Melting point | −38 °C; −36 °F; 235 K ([1]) |
| Boiling point | 211 °C; 412 °F; 484 K ([1]) |
| Solubility in water | Practically insoluble[1] |
| Solubility in octanol | Soluble |
| Refractive index (nD) | 1.418-1.421 |
| Hazards | |
| NFPA 704 |
 2
1
0
|
| Flash point | 83 °C (181 °F)[1] |
| LD50 | 3000 mg/kg (oral, rat)[2] 5000 mg/kg (dermal, rabbit)[2] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.[3]
Octyl acetate can be synthesized by a condensation of 1-octanol and acetic acid:
- CH3(CH2)7OH + CH3CO2H → CH3(CH2)7O2CCH3 + H2O
Uses
Because of its fruity odor, octyl acetate is used as the basis for artificial flavors and in perfumery. It is also as a solvent for nitrocellulose, waxes, oils, and some resins.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Record in the GESTIS Substance Database from the IFA
- ↑ 2.0 2.1 Food and Cosmetics Toxicology 12: 815. 1974.
- ↑ Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.