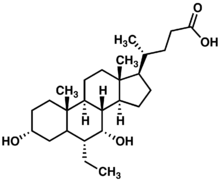
Obeticholic acid
 | |
|---|---|
| Systematic (IUPAC) name | |
| (3α,5β,6α,7α)-6-Ethyl-3,7-dihydroxycholan-24-oic acid
OR (4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)- 6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17- tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid | |
| Clinical data | |
| Trade names | Obeticholic acid |
| Legal status | Investigational |
| Routes | Oral |
| Identifiers | |
| CAS number | 459789-99-2 |
| ATC code | None |
| PubChem | CID 447715 |
| ChemSpider | 394730 |
| KEGG | C15636 |
| ChEMBL | CHEMBL566315 |
| Synonyms | 6α-ethyl-chenodeoxycholic acid; INT-747 |
| Chemical data | |
| Formula | C26H44O4 |
| Mol. mass | 420.62516 |
| SMILES
| |
| |
Obeticholic acid (abbreviated to OCA), is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It has also been known as INT-747. It is undergoing development as a pharmaceutical agent for several liver diseases and related disorders. Intercept Pharmaceuticals Inc. (NASDAQ symbol ICPT) hold the worldwide rights to develop OCA outside of Japan and China, where it is licensed to Dainippon Sumitomo Pharma.[1]
Invention and development
The natural bile acid, chenodeoxycholic acid, was identified in 1999 as the most active physiological ligand for the farnesoid X receptor (FXR), which is involved in many physiological and pathological processes. A series of alkylated bile acid analogues were designed, studied and patented by Roberto Pellicciari and colleagues at the University of Perugia, with 6α-ethyl-chenodeoxycholic acid emerging as the most highly potent FXR agonist.[2] FXR-dependent processes in liver and intestine were proposed as therapeutic targets in human diseases.[3] Obeticholic acid is the first FXR agonist to be used in human drug studies.
Clinical studies
OCA is undergoing development in phase 2 and 3 studies for specific liver and gastrointestinal disorders.[4]
Primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is an auto-immune, inflammatory liver disease of the liver which produces bile duct injury, fibrosis, cholestasis and eventual cirrhosis. It is much more common in women than men and can cause jaundice, itching (pruritus) and fatigue. Ursodeoxycholic acid therapy is beneficial, but the disease often progresses and may require liver transplantation.[5] Animal studies suggested that treatment with FXR agonists should be beneficial in cholestatic diseases such as PBC.[6] OCA was shown in two phase 2 studies to provide biochemical benefit.[7] A randomized, double-blind phase 3 study of OCA (5mg or 10mg compared to placebo), in combination with ursodeoxycholic acid, (POISE), is due to report in 2014.
Nonalcoholic steatohepatitis (NASH)
Non-alcoholic steatohepatitis is a common cause of abnormal liver function with histological features of fatty liver, inflammation and fibrosis. It may progress to cirrhosis and is becoming an increasing indication for liver transplantation. It is increasing in prevalence. OCA is proposed to treat NASH.[8] A phase 2 trial published in 2013 showed that administration of OCA at 25mg or 50mg daily for 6 weeks reduced markers of liver inflammation and fibrosis and increased insulin sensitivity.[9]
The Farnesoid X Receptor Ligand Obeticholic Acid in Nonalcoholic Steatohepatitis Treatment (FLINT) trial, sponsored by NIDDK, was halted early in January 2014, after about half of the 283 subjects had completed the study, when a planned interim analysis showed that the primary endpoint had been met. The treatment (OCA 25mg/day for 72 weeks) resulted in a highly statistically significant improvement (p=0.0024 on an intention-to-treat basis, compared to placebo) in the primary histological endpoint, defined as a decrease in the NAFLD Activity Score of at least two points, with no worsening of fibrosis.[10][11]
The market for the drug was estimated at $2 billion annually by 2020.[1]
Portal hypertension
Animal studies suggest that OCA improves intrahepatic vascular resistance and so may be of therapeutic benefit in portal hypertension.[12] An open label phase 2a clinical study is under way.
Bile acid diarrhea
Bile acid diarrhea (also called bile acid malabsorption) can be secondary to Crohn's disease or be a primary condition. Reduced median levels of FGF19, an ileal hormone that regulates increased hepatic bile acid synthesis, have been found in this condition.[13] FGF19 is potently stimulated by bile acids and especially by OCA.[14] A proof of concept study of OCA (25mg/d) has shown clinical and biochemical benefit.
References
- ↑ 1.0 1.1 Wall Street Journal. "A $4 Billion Surprise for 45-Person Biotech". Retrieved 10 January 2014.
- ↑ Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM (August 2002). "6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity". J. Med. Chem. 45 (17): 3569–72. doi:10.1021/jm025529g. PMID 12166927.
- ↑ Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S (September 2005). "Role of FXR in regulating bile acid homeostasis and relevance for human diseases". Curr. Drug Targets Immune Endocr. Metabol. Disord. 5 (3): 289–303. doi:10.2174/1568008054863781. PMID 16178789.
- ↑ "ClinicalTrials.gov".
- ↑ Hirschfield GM, Gershwin ME (January 2013). "The immunobiology and pathophysiology of primary biliary cirrhosis". Annu Rev Pathol 8: 303–30. doi:10.1146/annurev-pathol-020712-164014. PMID 23347352.
- ↑ Lindor, KD (2011 May). "Farnesoid X receptor agonists for primary biliary cirrhosis". Current opinion in gastroenterology 27 (3): 285–8. doi:10.1097/MOG.0b013e32834452c8. PMID 21297469.
- ↑ Fiorucci S, Cipriani S, Mencarelli A, Baldelli F, Bifulco G, Zampella A (August 2011). "Farnesoid X receptor agonist for the treatment of liver and metabolic disorders: focus on 6-ethyl-CDCA". Mini Rev Med Chem 11 (9): 753–62. doi:10.2174/138955711796355258. PMID 21707532.
- ↑ Adorini L, Pruzanski M, Shapiro D (September 2012). "Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis". Drug Discov. Today 17 (17–18): 988–97. doi:10.1016/j.drudis.2012.05.012. PMID 22652341.
- ↑ Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D (September 2013). "Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease". Gastroenterology 145 (3): 574–82.e1. doi:10.1053/j.gastro.2013.05.042. PMID 23727264.
- ↑ [http://clinicaltrial.gov/ct2/show/NCT01265498. "The Farnesoid X Receptor (FXR) Ligand Obeticholic Acid in NASH Treatment Trial(FLINT)"].
- ↑ Intercept Pharma. "Press release". Retrieved 9 January 2014.
- ↑ Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Vander Elst I, Windmolders P, Vanuytsel T, Nevens F, Laleman W (November 2013). "Obeticholic acid, a farnesoid-X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats". Hepatology: n/a. doi:10.1002/hep.26939. PMID 24259407.
- ↑ Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW (November 2009). "A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis". Clin. Gastroenterol. Hepatol. 7 (11): 1189–94. doi:10.1016/j.cgh.2009.04.024. PMID 19426836.
- ↑ Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, Williamson C, Walters JR (May 2013). "Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids". Am. J. Physiol. Gastrointest. Liver Physiol. 304 (10): G940–8. doi:10.1152/ajpgi.00398.2012. PMC 3652069. PMID 23518683.
External links
| |||||||||||||