Nystatin
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
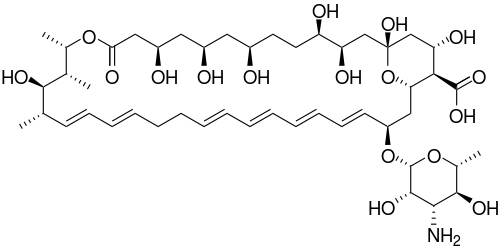
| (1S,3R,4R,7R,9R,11R,15S,16R,17R,18S,19E,21E, 25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,
6-dideoxy-β-L-mannopyranosyl)oxy]-1, 3,4,7,9,11,17,37-octahydroxy-15, 16,18-trimethyl-13-oxo-14, 39-dioxabicyclo[33.3.1]nonatriaconta-19, 21,25,27,29,31-hexaene-36-carboxylic acid | |
| Clinical data | |
| Trade names | generics only |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682758 |
| Pregnancy cat. | C |
| Legal status | ℞ Prescription only |
| Routes | topical & oral (but not absorbed) |
| Pharmacokinetic data | |
| Bioavailability | 0% on oral ingestion |
| Metabolism | None (not extensively absorbed) |
| Half-life | Dependent upon GI transit time |
| Excretion | Fecal (100%) |
| Identifiers | |
| CAS number | 1400-61-9 |
| ATC code | A07AA02 D01AA01 G01AA01 |
| PubChem | CID 14960 |
| DrugBank | DB00646 |
| ChemSpider | 23078586 |
| UNII | BDF1O1C72E |
| KEGG | D00202 |
| ChEBI | CHEBI:473992 |
| ChEMBL | CHEMBL229383 |
| NIAID ChemDB | 004993 |
| Chemical data | |
| Formula | C47H75NO17 |
| Mol. mass | 926.09 |
| SMILES
| |
| |
| | |
Nystatin (originally named Fungicidin) is a polyene antifungal medication to which many molds and yeast infections are sensitive, including Candida. Due to its toxicity profile, there are currently no injectable formulations of this drug on the US market.[1] However, nystatin may be safely given orally as well as applied topically due to its minimal absorption through mucocutaneous membranes such as the gut and the skin.[2][3]
Uses
Cutaneous, vaginal, mucosal and esophageal Candida infections usually respond well to treatment with nystatin. Cryptococcus is also sensitive to nystatin. In the UK its licence for treating neonatal oral thrush is restricted to those over the age of one month (miconazole is an appropriate alternative for younger babies). [citation needed]
Nystatin is often used as prophylaxis in patients who are at risk for fungal infections, such as AIDS patients with a low CD4+ count and patients receiving chemotherapy.
It is prescribed in units, with doses varying from 100,000 (for oral infections) to 1 million (for intestinal ones). As it is not absorbed from the gut, it is safe for oral use and does not have problems of drug interactions.
It is also used in cellular biology as an inhibitor of the lipid raft-caveolae endocytosis pathway on mammalian cells, at concentrations around 3 µg/mL.

In certain cases, nystatin has been used to prevent the spread of mold on objects such as works of art. For example, it was applied to wood panel paintings damaged as a result of the Arno River Flood of 1966 in Florence, Italy.
Nystatin is also used as a tool by scientists performing "perforated" patch-clamp electrophysiologic recordings of cells. When loaded in the recording pipette, it allows for measurement of electrical currents without washing out the intracellular contents, because it forms pores in the cell membrane that are permeable to only monovalent ions.[4]
Mechanism of action
Like amphotericin B and natamycin, nystatin binds to ergosterol, a major component of the fungal cell membrane. When present in sufficient concentrations, it forms pores in the membrane that lead to K+ leakage and death of the fungus. Ergosterol is fairly unique to fungi, so the drug does not have such catastrophic effects on animals or plants.
Origin

Like many other antifungals and antibiotics, nystatin is of bacterial origin. It was isolated from Streptomyces noursei in 1950 by Elizabeth Lee Hazen and Rachel Fuller Brown, who were doing research for the Division of Laboratories and Research of the New York State Department of Health. Hazen found a promising micro-organism in the soil of a friend's dairy farm. She named it Streptomyces noursei, after William Nourse, the farm's owner.[5] Hazen and Brown named nystatin after the New York State Health Department Laboratory (now known as the Wadsworth Center) in 1954.
Brand names
- Nystan (oral tablets, topical ointment, and pessaries, formerly from Bristol-Myers Squibb)
- Infestat
- Nystalocal from Medinova AG
- Nystamont
- Nystop (topical powder, Paddock)
- Nystex
- Mykinac
- Nysert (vaginal suppositories, Procter & Gamble)
- Nystaform (topical cream, and ointment and cream combined with iodochlorhydroxyquine and hydrocortisone; formerly Bayer now Typharm Ltd)
- Nilstat (vaginal tablet, oral drops, Lederle)
- Korostatin (vaginal tablets, Holland Rantos)
- Mycostatin (vaginal tablets, topical powder, suspension Bristol-Myers Squibb)
- Mycolog-II (topical ointment, combined with triamcinolone; Apothecon)
- Mytrex (topical ointment, combined with triamcinolone)
- Mykacet (topical ointment, combined with triamcinolone)
- Myco-Triacet II (topical ointment, combined with triamcinolone)
- Flagystatin II (cream, combined with metronidazole)
- Nistatina (oral tablets, Antibiotice Iaşi)
- Nidoflor (cream, combined with neomycin sulfate and triamcinolone acetonide)
- Stamicin (oral tablets, Antibiotice Iaşi)
- Lystin
- Animax (veterinary topical ointment or cream; combined with neomycin sulfate, thiostrepton and triamcinolone acetonide)
References
- ↑ Nystatin on RxList.com http://www.rxlist.com/script/main/srchcont_rxlist.asp?src=nystatin
- ↑ Nystatin on Micromedex http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/BCFBA8/DUPLICATIONSHIELDSYNC/6B2A9E/ND_PG/PRIH/ND_B/HCS/SBK/3/ND_P/Main/PFActionId/hcs.common.RetrieveDocumentCommon/DocId/0105/ContentSetId/31#pharmacokineticsSection
- ↑ Nystatin on UpToDate http://www.utdol.com/online/content/topic.do?topicKey=drug_l_z/184320&selectedTitle=1%7E44&source=search_result#F202808
- ↑ Akaike N, Harata N (1994). "Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms" (). Jpn. J. Physiol. 44 (5): 433–73. doi:10.2170/jjphysiol.44.433. PMID 7534361.
- ↑ Ana Espinel-Ingroff, Medical mycology in the United States: a historical analysis (1894-1996), Springer, 2003, p. 62.
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||