Nutlin
| Nutlin | ||
|---|---|---|
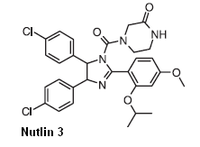 | ||
| IUPAC name (±)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one Nutlin-3 | ||
| Other names Nutlin | ||
| Identifiers | ||
| CAS number | 548472, (Nutlin-3) | |
| PubChem | 16755649 | |
| ChEMBL | CHEMBL191334 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C30H30Cl2N4O4 | |
| Molar mass | 581.4896 [g/mol] | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Nutlins are cis-imidazoline analogs which inhibit the interaction between mdm2 and tumour suppressor p53, and were discovered by screening a chemical library by Vassilev et al. Nutlin-1, Nutlin-2 and Nutlin-3 were all identified in the same screen,[1] however Nutlin-3 is the compound most commonly used in anti-cancer studies.[2] Inhibiting the interaction between mdm2 and p53 stabilizes p53 and is thought to selectively induce a growth-inhibiting state called senescence in cancer cells. These compounds are therefore thought to work best on tumors that contain normal or wild type p53.[citation needed] Nutlin-3 has been shown to affect the production of p53 within minutes.[3]
The more potent of the two enantiomers, (-)-Nutlin-3, can now be synthesized in a highly enantioselective fashion.[4]
References
- ↑ Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004). "In vivo activation of the p53 pathway by small-molecule antagonists of MDM2". Science 303 (5659): 844–848. doi:10.1126/science.1092472. PMID 14704432.
- ↑ Shangary S, Wang S. (2008). "Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction to Reactivate p53 Function: A Novel Approach for Cancer Therapy". Annu Rev Pharmacol Toxicol. 49: 223–241. doi:10.1146/annurev.pharmtox.48.113006.094723. PMC 2676449. PMID 18834305.
- ↑ Ingeborg M.M. van Leeuwen, Maureen Higgins, Johanna Campbell, Christopher J. Brown, Anna R. McCarthy, Lisa Pirrie, Nicholas J. Westwood and Sonia Laín (15 May 2011). "Mechanism-specific signatures for small-molecule p53 activators". Cell Cycle (Landes Bioscience) 10 (10).
- ↑ Tyler A. Davis, Jeffrey N. Johnston. Catalytic, enantioselective synthesis of stilbene cis-diamines: A concise preparation of (-)-Nutlin-3, a potent p53/MDM2 inhibitor.Chemical Science. The Royal Society of Chemistry. 2, February 2011.