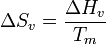Nucleation

Nucleation is the process of forming a nucleus. It is the initial process in crystallization. Nucleation is the extremely localized budding of a distinct thermodynamic phase. It is the process in which ions, atoms, or molecules arrange themselves in a pattern characteristic of a crystalline solid, forming a site in which additional particles deposit as the crystal grows. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals, or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation (see Cloud condensation nuclei). Nucleation of crystalline, amorphous, and even vacancy clusters solid materials is also important, for example to the semiconductor industry. Most nucleation processes are physical rather than chemical, but a few exceptions do exist (e.g., electrochemical nucleation).
A good example is the famous Diet Coke and Mentos eruption. Nucleation normally occurs at nucleation sites on surfaces contacting the liquid or vapor. Suspended particles or minute bubbles also provide nucleation sites. This is called heterogeneous nucleation. Nucleation without preferential nucleation sites is homogeneous nucleation. Homogeneous nucleation occurs spontaneously and randomly, but it requires superheating or supercooling of the medium. Nucleation is involved in such processes as cloud seeding and in instruments such as the bubble chamber and the cloud chamber.
Examples of nucleation
- Clouds form when wet air cools (often because the air is rising) and many small water droplets nucleate from the supersaturated air.[1] The amount of water that can exist in air as a vapour decreases on cooling and cooling air can result in there being too much water vapour for the air to hold, driving the nucleation of small water droplets which form a cloud. Nucleation of the droplets of liquid water is heterogeneous, it occurs on particles referred to as cloud condensation nuclei, and cloud formation can be altered by adding artificial cloud condensation nuclei, this is called cloud seeding.
- Nucleation is by definition the first step in crystallisation, and so it determines if a crystal can form. Frequently crystals do not form even when they are thermodynamically the favoured state. For example small droplets of very pure water can remain liquid down to below -30 C although ice is the stable state below 0 C.[1]

- Bubbles of carbon dioxide nucleate shortly after the pressure is released from a container of carbonated liquid. Nucleation often occurs more easily at a pre-existing interface (heterogeneous nucleation), as happens on boiling chips and string used to make rock candy. The so-called Diet Coke and Mentos eruption is a dramatic example.
- Nucleation in boiling can occur in the bulk liquid if the pressure is reduced so that the liquid becomes superheated with respect to the pressure-dependent boiling point. More often, nucleation occurs on the heating surface, at nucleation sites. Typically, nucleation sites are tiny crevices where free gas-liquid surface is maintained or spots on the heating surface with lower wetting properties. Substantial superheating of a liquid can be achieved after the liquid is de-gassed and if the heating surfaces are clean, smooth and made of materials well wetted by the liquid.
- Nucleation is relevant in the process of crystallization of nanometer sized materials,[2] and plays an important role in atmospheric processes.
- Nucleation is a key concept in polymer,[3] alloy and ceramic systems.
- In chemistry and biophysics, nucleation is the phaseless formation of multimers, which are intermediates in polymerization processes. This sort of process is believed to be the best model for processes such as crystallization and amyloidogenesis.
- In molecular biology, nucleation is the critical stage in the assembly of a polymeric structure, such as a microfilament, at which a small cluster of monomers aggregates in the correct arrangement to initiate rapid polymerization. For instance, two actin molecules bind weakly, but addition of a third stabilizes the complex. This trimer then adds additional molecules and forms a nucleation site. The nucleation site serves the slow, or lag phase of the polymerization process.
- Some champagne stirrers operate by providing many nucleation sites via high surface area and sharp corners, speeding the release of bubbles and removing carbonation from the wine.
- Sodium acetate heating pads use cavitation voids caused by the deflection of a metal disk as nucleation centres for exothermic crystallization.
Mechanics of nucleation
_from_a_metastable_phase_(white)_in_the_Ising_model.ogg.jpg)
Nucleation is a stochastic process, i.e., there is a random element to it and so in a set of identical droplets nucleation will occur over a spread of times. If the nucleation rate is constant then the fraction of these droplets where nucleation has not occurred should decrease exponentially with time. This is seen for example in the nucleation of ice in supercooled small water droplets.[4] The decay rate of the exponential gives the nucleation rate.
Homogeneous nucleation
In general, nucleation occurs with much more difficulty in the interior of a uniform substance, by a process called homogeneous nucleation. The creation of a nucleus implies the formation of an interface at the boundaries of a new phase.
Liquids cooled below the maximum heterogeneous nucleation temperature (melting temperature) but are above the homogeneous nucleation temperature (pure substance freezing temperature) are said to be supercooled. This is useful for making amorphous solids and other metastable structures but can delay the progress of industrial chemical processes or produce undesirable effects in the context of casting.
Supercooling brings about supersaturation, the driving force for nucleation. Supersaturation occurs when the pressure in the newly formed solid is less than the liquid pressure, and brings about a change in free energy per unit volume,  , between the liquid and newly created solid phase. This change in free energy is balanced by the energy gain of creating a new volume, and the energy cost due to creation of a new interface. When the overall change in free energy,
, between the liquid and newly created solid phase. This change in free energy is balanced by the energy gain of creating a new volume, and the energy cost due to creation of a new interface. When the overall change in free energy, 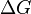 is negative, nucleation is favored.
is negative, nucleation is favored.
Some energy is consumed to form an interface, based on the surface energy of each phase. If a hypothetical nucleus is too small (known as an unstable nucleus or "embryo"), the energy that would be released by forming its volume is not enough to create its surface, and nucleation does not proceed. The critical nucleus size can be denoted by its radius, and it is when r=r* (or r critical) that the nucleation proceeds.
For example,in the classic case[5] of a spherical cluster that liberates  Joules per cubic centimeter during formation (here
Joules per cubic centimeter during formation (here  is a negative quantity) but must pay the positive cost of σ Joules per square centimeter of surface interfacing with the surrounding, the free energy needed to form a spherical cluster of radius r is
is a negative quantity) but must pay the positive cost of σ Joules per square centimeter of surface interfacing with the surrounding, the free energy needed to form a spherical cluster of radius r is
where the first term shows the energy gain of creating a new volume and the second term shows the energy loss due to surface tension (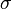 ) of the new interface.
) of the new interface.
It costs free energy to add molecules to this cluster (because  ) until the radius reaches
) until the radius reaches
where

Addition of new molecules to clusters larger than this critical radius releases, rather than costs, available work. In other words, at that point, growth of the cluster is no longer limited by nucleation but perhaps by diffusion[6] (i.e., the supply of molecules) or by reaction kinetics instead.
The free energy needed to form this critical radius can be found by
which occurs at the maximum 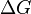 where
where 
The term  can be related to the equilibrium temperature, enthalpy of fusion (
can be related to the equilibrium temperature, enthalpy of fusion ( ), and the degree of undercooling (
), and the degree of undercooling ( ) as follows,
) as follows,
By evaluating this equation at the equilibrium point ( ) at the melting temperature
) at the melting temperature  we achieve,
we achieve,
Substitution of 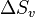 into the first equation leads to
into the first equation leads to
Which by using common denominators and the definition of 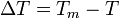 provides
provides
As the phase transformation becomes more and more favorable, the formation of a given volume of nucleus frees enough energy to form an increasingly large surface, allowing progressively smaller nuclei to become viable. Eventually, thermal activation will provide enough energy to form stable nuclei. These can then grow until thermodynamic equilibrium is restored.
A greater degree of supercooling favors phase transformation, and we can relate 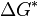 to supercooling and find r* and
to supercooling and find r* and 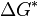 as a function of
as a function of  by the substitution of
by the substitution of 
and
The greater the supercooling, the smaller the critical radius and the less energy needed to form it.
The spontaneous nucleation rate in, say, water changes very rapidly with temperature, so the spontaneous nucleation temperature can be quite well-defined. 'Film boiling' on very hot surfaces and the Leidenfrost effect are both believed to be stabilized by spontaneous nucleation phenomena.
Heterogeneous nucleation

Heterogeneous nucleation, nucleation with the nucleus at a surface, is much more common than homogeneous nucleation. Heterogeneous nucleation is typically much faster than homogeneous nucleation because the nucleation barrier ΔG* is much lower at a surface. This is because the nucleation barrier comes from the positive term in the free energy ΔG, which is the surface term. For homogeneous nucleation the nucleus is approximated by a sphere and so has a free energy equal to the surface area of a sphere, 4πr2, times the surface tension σ. However, as we can see in the schematic of macroscopic droplets to the right, droplets on surfaces are not complete spheres and so the area of the interface between the droplet and the surrounding fluid is less than 4πr2. This geometrical factor reduces the interfacial area and so the interfacial free energy, which in turn reduces the nucleation barrier.[7] Note that this simple theory treats the microscopic nucleus just as if it is a macroscopic droplet.
In the schematic to the right the contact angle between the droplet surface and the surface decreases from left to right (A to C), and we see that the surface area of the droplet decreases as the contact angle decreases. This geometrical effect reduces the barrier and hence results in faster nucleation on surfaces with smaller contact angles. Also, if instead of the surface being flat it curves towards fluid, then this also reduces the interfacial area and so the nucleation barrier. There are expressions for this reduction for simple surface geometries.[8] In practice, this means we expect nucleation to be fastest on pits or cracks in surfaces made of material such that the nucleus forms a small contact angle on its surface.

Similar effects can cause precipitate particles to form at the grain boundaries of a solid. This can interfere with precipitation strengthening, which relies on homogeneous nucleation to produce a uniform distribution of precipitate particles.
Rate of nucleation
The nucleation rate, I, depends on the average number of critical clusters, n* and the diffusion of molecules to the cluster,  .
.
where the average population of critical nuclei is
where
- ΔG* is critical free energy needed corresponding to that of the critical radius.
- N is the number of potential nucleation sites per unit volume
- kB is the Boltzmann constant
The number of clusters of a certain size formed is a function of the total number of atoms in the system, the free energy to create a cluster (of that size), and the temperature. The number of clusters increases with increasing temperature.
Plugging in for  , we get
, we get
The rate of addition of one extra atom to the critical nucleus as estimated by the Volmer-Weber theory is
where A is a term that incorporates the shape factor of the atoms and the area to which atoms can join, and the vibrational frequency of the particles. And Q is the activation energy for atomic migration.
This term gives us the diffusion of the atoms to the site of nucleation. However, a problem with the Volmer Weber theory is that it ignores formation of particles of r>r* and assumes that size distribution is maintained (fluctuations are occurring fast)
The rate of nucleation can be expressed as
where
- γ is the surface tension.
- ΔHs is the enthalpy per unit volume.
- Tm is the melting temperature.
- Θ is the wetting angle.

At very low temperatures, rate of diffusion is low. As temperature increases, the rate of diffusion increases; molecules are able to get to the site of nucleation at a fast enough rate to promote growth of the nucleus. At temperatures significantly below melting temperature, fluctuation of molecules is very low; the molecules are in a low energy state and do not have enough energy to move around and nucleate. Nucleation rate is dominated by diffusion. However, as temperature increases, molecular fluctuations increase and molecules tend to escape from the nucleus, causing a decreased rate of nucleation.
The time required for steady state nucleation is known as the time-lag  and can be found by[9]
and can be found by[9]
Where:
- a is the average particle size.
- h is Planck's constant.
The spinodal region
Phase transition processes can also be explained in terms of spinodal decomposition, where phase separation is delayed until the system enters the unstable region where a small perturbation in composition leads to a decrease in energy and, thus, spontaneous growth of the perturbation.[10] This region of a phase diagram is known as the spinodal region and the phase separation process is known as spinodal decomposition and may be governed by the Cahn–Hilliard equation.
Modern theory
Classical nucleation
Because of the many unjustified assumptions made by the classical nucleation theory (CNT), it has limited applicability to the solution of practical problems when compared with experimental data. The limitations arise mainly because the CNT assumes that macroscopic properties of molecules can be applied to microscopic activities. This can be a major drawback when dealing with characteristics such as density, surface tension, and saturated vapour pressure of clusters consisting of only several tens of molecules. The classical nucleation theory also does not take into consideration the interaction of particles around the nuclei, which leads to thermodynamics.
Computer simulation studies of simple models
The classical nucleation theory makes a number of assumptions, for example it treats a microscopic nucleus as if it is a macroscopic droplet with a well defined surface whose free energy is estimated using an equilibrium property: the interfacial tension σ. Nucleation is an inherently out of thermodynamic equilibrium phenomenon so it is not always obvious that its rate can estimated using equilibrium properties.
However, modern computers are powerful enough to calculate essentially exact nucleation rates for simple models. These have been compared with the classical theory, for example for the case of nucleation of the crystal phase in the model of hard spheres. This is a model of perfectly hard spheres in thermal motion, and is a simple model of some colloids. For the crystallization of hard spheres the classical theory is a very reasonable approximate theory.[11]
Modern technology
Nucleation is a topic of wide interest in many scientific studies and technological processes. It is used heavily in the chemical industry for cases such as in the preparation of metallic ultradispersed powders that can serve as catalysts. For example, platinum deposited onto TiO2 nanoparticles catalyses the liberation of hydrogen from water.[12] It is also an important factor in the semiconductor industry, as the gap width in semiconductors is influenced by the size of metal nanoclusters.[13]
Experiment
It is typically difficult to experimentally study nucleation. The nucleus is microscopic (its size is r* above) so the nucleus is too small to be directly observed. In large liquid volumes there are typically multiple nucleation events and it is difficult to disentangle the effects of nucleation from those of growth of the nucleated phase. These problems can be got around by working with small droplets. As nucleation is stochastic, many droplets are needed so that statistics for the nucleation events can be obtained..png)
Nucleation occurs in different droplets at different times, hence the fraction is not a simple step function that drops sharply from one to zero at one particular time. The red curve is a fit of a Gompertz function to the data. This is a simplified version of the model Pound and La Mer used to model their data.[14] The model assumes that nucleation occurs due to impurity particles in the liquid tin droplets, and it makes the simplifying assumption that all impurity particles produce nucleation at the same rate. It also assumes that these particles are Poisson distributed among the liquid tin droplets. The fit values are that the nucleation rate due to a single impurity particle is 0.02/s, and the average number of impurity particles per droplet is 1.2. Note that about 30% of the tin droplets never freeze; the data plateaus at a fraction of about 0.3. Within the model this is assumed to be because, by chance, these droplets do not have even one impurity particle and so there is no heterogeneous nucleation. Homogeneous nucleation is assumed to be negligible on the timescale of this experiment. The remaining droplets freeze in a stochastic way, at rates 0.02/s if they have one impurity particle, 0.04/s if they have two, and so on.
This data is just one example but it does illustrate common features of the nucleation of crystals in that there is clear evidence for heterogeneous nucleation, and that nucleation is clearly stochastic.
Gustav Tammann developed a method to study nucleation, known as the Tammann or “development” method.[15] In this method, crystals are nucleated at a low temperature Tn and then grown at a higher temperature Tg. For validity of this method, the nucleation rate I has to be greater at the nucleation temperature Tn than at the growth temperature Tg; I(Tn)>> I(Tg), and the growth rate U must be greater at the growth temperature than at the nucleation temperature Tn; U(Tg) >> U(Tn). Since the clusters are heated to a larger temperature with a larger critical radius requirement, clusters no longer meet the critical radius requirement and remelt. A method to heat the particles carefully must be used.
Koster proposed a method for nucleation of metallic glasses.[9] This method considers the sizes of different crystals and attempts to determine when they were formed using data of their growth rates. It can be used for both homogeneous and heterogeneous nucleation.
References
- ↑ 1.0 1.1 H. R. Pruppacher and J. D. Klett, Microphysics of Clouds and Precipitation, Kluwer (1997).
- ↑ Mendez-Villuendas, Eduardo; Bowles, Richard (2007). "Surface Nucleation in the Freezing of Gold Nanoparticles". Physical Review Letters 98 (18). arXiv:cond-mat/0702605. doi:10.1103/PhysRevLett.98.185503.
- ↑ Young, R. J. (1981) Introduction to Polymers (CRC Press, NY) ISBN 0-412-22170-5.
- ↑ Duft, D.; Leisner (2004). "Laboratory evidence for volume-dominated nucleation of ice in supercooled water microdroplets". Atmospheric Chemistry & Physics 4: 1997. doi:10.5194/acp-4-1997-2004.
- ↑ F. F. Abraham (1974) Homogeneous nucleation theory (Academic Press, NY).
- ↑ Ham, Frank S. (1959). "Diffusion-Limited Growth of Precipitate Particles". Journal of Applied Physics 30 (10): 1518. doi:10.1063/1.1734993.
- ↑ Sear, R. P. (2007). "Nucleation: theory and applications to protein solutions and colloidal suspensions". J. Physics Cond. Matt. 19 (3): 033101. doi:10.1088/0953-8984/19/3/033101.
- ↑ Sholl, C. A.; N. H. Fletcher (1970). "Decoration criteria for surface steps". Acta Metall. 18: 1083. doi:10.1016/0001-6160(70)90006-4.
- ↑ 9.0 9.1 Fokin, Vladimir M.; Yuritsyn, Nikolay S. and Zanotto, Edgar D. (2005) "Nucleation and Crystallization Kinetics in Silicate Glasses: Theory and Experiment", pp. 76–83 in Schmelzer, J (Ed.) Nucleation Theory and Applications, Wiley, doi:10.1002/3527604790.ch4, ISBN 9783527404698.
- ↑ Mendez-Villuendas, Eduardo; Saika-Voivod, Ivan; Bowles, Richard K. (2007). "A limit of stability in supercooled liquid clusters". The Journal of Chemical Physics 127 (15): 154703. doi:10.1063/1.2779875. PMID 17949187.
- ↑ Auer, S.; D. Frenkel (2004). "Numerical prediction of absolute crystallization rates in hard-sphere colloids". J. Chem. Phys. 120: 3015. doi:10.1063/1.1638740.
- ↑ Palmans, Roger; Frank, Arthur J. (1991). "A molecular water-reduction catalyst: Surface derivatization of titania colloids and suspensions with a platinum complex". The Journal of Physical Chemistry 95 (23): 9438. doi:10.1021/j100176a075.
- ↑ Rajh, Tijana; Micic, Olga I.; Nozik, Arthur J. (1993). "Synthesis and characterization of surface-modified colloidal cadmium telluride quantum dots". The Journal of Physical Chemistry 97 (46): 11999. doi:10.1021/j100148a026.
- ↑ 14.0 14.1 Pound, Guy M.; V. K. La Mer (1952). "Kinetics of Crystalline Nucleus Formation in Supercooled Liquid Tin". J. American Chemical Society 74: 2323. doi:10.1021/ja01129a044.
- ↑ Tammann, G. (1898). "Über die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur". Zeits. f. Physik. Chemie 25: 441.