Novec 1230
| Novec 1230 | |
|---|---|
 |
 |
| Other names perfluoro(2-methyl-3-pentanone), heptafluoroisopropyl pentafluoroethyl ketone, | |
| Identifiers | |
| CAS number | 756-13-8 |
| PubChem | 2782408 |
| ChemSpider | 2062563 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6F12O |
| Molar mass | 316.04 g mol−1 |
| Density | 1.723 g/cm3 |
| Melting point | −108 °C; −162 °F; 165 K |
| Boiling point | 49 °C; 120 °F; 322 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Novec 1230, C6F12O, (3M Novec 1230) fluid is a low global warming potential Halon replacement for use as a gaseous fire suppression agent. Novec 1230 is manufactured by 3M. It is generally used in situations where water from a fire sprinkler would damage expensive equipment or where water-based fire protection is impractical, such as museums, banks, clean rooms and hospitals.
3M Novec 1230 fluid does not deplete ozone (ODP 0) and has a global warming potential of 1 over 100 years.[1]
Novec 1230 fluid is a high molecular weight material, compared with the first generation halocarbon clean agents. The product has a heat of vaporization of 88.1 kJ/kg and low vapor pressure. Although it is a liquid at room temperature it gasifies immediately after being discharged in a total flooding system.
The product is ideal for use in total flooding applications, localized flooding systems, directional spray type applications and may be used in portable extinguishers for specialized applications. But in addition to the conventional methods of super-pressurization using nitrogen, Novec 1230 fluid also lends itself for use in pump applications because it is a liquid.
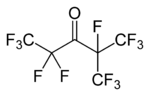
Chemically, it is a fluorinated ketone with the systematic name 1,1,1,2,2,4,5,5,5-nonafluoro-4-(trifluoromethyl)-3-pentanone and the structural formula CF3CF2C(=O)CF(CF3)2, a fully fluorinated analog of ethyl isopropyl ketone.
See also
- Fire Protection Fluid