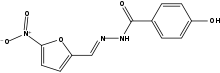
Nifuroxazide
 | |
|---|---|
| Systematic (IUPAC) name | |
| 4-Hydroxy-N'-[(5-nitrofuran-2-yl)methylene]benzohydrazide | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | ? |
| Routes | Oral |
| Identifiers | |
| CAS number | 965-52-6 |
| ATC code | A07AX03 |
| PubChem | CID 5337997 |
| ChemSpider | 4495115 |
| UNII | PM5LI0P38J |
| KEGG | D07111 |
| ChEMBL | CHEMBL244888 |
| Chemical data | |
| Formula | C12H9N3O5 |
| Mol. mass | 275.2 g/mol |
| SMILES
| |
| |
| | |
Nifuroxazide (INN) is an oral nitrofuran antibiotic, patented since 1966[1] and used to treat colitis and diarrhea in humans and non-humans.[2] It is sold under the brand names Ambatrol, Antinal, Bacifurane, Diafuryl (France), Diax (Egypt), Nifrozid, Ercefuryl (Romania, Czech Republic), Erfuzide (Thailand), Endiex (Slovakia), Nifuroksazyd (Poland), Pérabacticel (France), Pentofuryl (Germany), Topron (Latin America), Antinal (Egypt), Apazid (Morocco) and Septidiaryl. It is sold in capsule form and also as a suspension. The pharmaceutical group GlaxoSmithKline plc (Previously known as SmithKline Beecham) claims that nifuroxazide is highly effective and the consumers' group Healthy Skepticism says that GlaxoSmithKline's claims have no scientific support.[3]
History
Maurice Claude Ernest Carron patented the drug in the United States in 1966.[1] Subsequent patents issued to Germano Cagliero of Marxer S.p.A describe the use of nifuroxazide as an antibiotic used to treat livestock.[2]
Effectiveness in humans
In 1997, in an Ivory Coast promotional leaflet, GlaxoSmithKline claimed that nifuroxazide (under the brand name "Ambatrol") is an anti-dehydration treatment, "neutralise[s] microbacterials" in diarrhoea, and has "a spectrum which covers most enteropathogenic microbacterials, Shigella, Escherichia coli, Salmonella, Staphylococci, Klebsiella, Yersinia".[3] The international non-profit organisation Healthy Skepticism, at the time using their former name, Medical Lobby for Appropriate Marketing (MaLAM), disagreed, stating "We have not found any scientific evidence to support these claims."[3]
Notes
- ↑ 1.0 1.1 USPTO No. 3290213 |http://www.google.com/patents?id=f2dwAAAAEBAJ
- ↑ 2.0 2.1 USPTO No 4093746 |http://www.google.com/patents?vid=USPAT4093746
- ↑ 3.0 3.1 3.2 "SmithKline Beecham Ambatrol (nifuroxazide)". Healthy Skepticism. June 1997. Archived from the original on 2010-12-21. Retrieved 2010-12-21.
| ||||||||||||||||||||||||||||