Nickel titanium
Nickel titanium, also known as nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages.
| Material properties | |
|---|---|
| Density | 6.45 g/cm3 |
| Electrical Resistivity (Austenite) |
 |
| (Martensite) |
 |
| Thermal Conductivity (Austenite) | 0.18 W/cm·K |
| (Martensite) | 0.086 W/cm·K |
| Coefficient of Thermal Expansion (Austenite) |
 |
| (Martensite) |
 |
| Magnetic Permeability | < 1.002 |
| Magnetic Susceptibility (Austenite) |
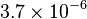 emu/g emu/g |
| (Martensite) |
 emu/g emu/g |
| Elastic Modulus (Austenite) | 75-83 GPa |
| (Martensite) | 28-40 GPa |
| Yield Strength (Austenite) | 195-690 MPa |
| (Martensite) | 70-140 MPa |
| Poisson's Ratio | 0.33 |
| Nitinol properties are particular to the precise composition of the alloy and its processing. These specifications are typical for commercially available shape memory nitinol alloys. | |
Nitinol alloys exhibit two closely related and unique properties: shape memory and superelasticity (also called pseudoelasticity). Shape memory is the ability of nitinol to undergo deformation at one temperature, then recover its original, undeformed shape upon heating above its "transformation temperature". Superelasticity occurs at a narrow temperature range just above its transformation temperature; in this case, no heating is necessary to cause the undeformed shape to recover, and the material exhibits enormous elasticity, some 10-30 times that of ordinary metal.
History
The term nitinol is derived from its composition and its place of discovery: (Nickel Titanium-Naval Ordnance Laboratory). William J. Buehler[1] along with Frederick Wang,[2] discovered its properties during research at the Naval Ordnance Laboratory in 1959.[3][4]
While the potential applications for nitinol were realized immediately, practical efforts to commercialize the alloy did not take place until a decade later. This delay was largely because of the extraordinary difficulty of melting, processing and machining the alloy. Even these efforts encountered financial challenges that were not really overcome until the 1990s, when these practical difficulties finally began to be resolved.
The discovery of the shape-memory effect in general dates back to 1932, when Swedish chemist Arne Ölander [5] first observed the property in gold-cadmium alloys. The same effect was observed in Cu-Zn in the early 1950s.[6]
Mechanism

Nitinol's unusual properties are derived from a reversible solid-state phase transformation known as a martensitic transformation, between two different martensite crystal phases, requiring 10,000–20,000 psi (69–138 MPa) of mechanical stress.
At high temperatures, nitinol assumes an interpenetrating primitive cubic crystal structure referred to as austenite (also known as the parent phase). At low temperatures, nitinol spontaneously transforms to a more complicated monoclinic crystal structure known as martensite (daughter phase). The temperature at which austenite transforms to martensite is generally referred to as the transformation temperature. More specifically, there are four transition temperatures. When the alloy is fully austenite, martensite begins to form as the alloy cools at the so-called martensite start, or Ms temperature, and the temperature at which the transformation is complete is called the martensite finish, or Mf temperature. When the alloy is fully martensite and is subjected to heating, austenite starts to form at the As temperature, and finishes at the Af temperature.[7]

Crucial to nitinol properties are two key aspects of this phase transformation. First is that the transformation is "reversible", meaning that heating above the transformation temperature will revert the crystal structure to the simpler austenite phase. The second key point is that the transformation in both directions is instantaneous.
Martensite's crystal structure (known as a monoclinic, or B19' structure) has the unique ability to undergo limited deformation in some ways without breaking atomic bonds. This type of deformation is known as twinning, which consists of the rearrangement of atomic planes without causing slip, or permanent deformation. It is able to undergo about 6–8% strain in this manner. When martensite is reverted to austenite by heating, the original austenitic structure is restored, regardless of whether the martensite phase was deformed. Thus the name "shape memory" refers to the fact that the shape of the high temperature austenite phase is "remembered", even though the alloy is severely deformed at a lower temperature.[8]
A great deal of force can be produced by preventing the reversion of deformed martensite to austenite — from 35,000 psi to, in many cases, more than 100,000 psi (689 MPa). One of the reasons that nitinol works so hard to return its original shape is that it is not just an ordinary metal alloy, but what is known as an intermetallic compound. In an ordinary alloy, the constituents are randomly positioned on the crystal lattice; in an ordered intermetallic compound, the atoms (in this case, nickel and titanium) have very specific locations in the lattice.[9] The fact that nitinol is an intermetallic is largely responsible for the difficulty in fabricating devices made from the alloy.

The scenario described above (cooling austenite to form martensite, deforming the martensite, then heating to revert to austenite, thus returning the original, undeformed shape) is known as the thermal shape memory effect. To fix the original "parent shape", the alloy must be held in position and heated to about 500 °C. A second effect, called superelasticity or pseudoelasticity, is also observed in nitinol. This effect is the direct result of the fact that martensite can be formed by applying a stress as well as by cooling. Thus in a certain temperature range, one can apply a stress to austenite, causing martensite to form while at the same time changing shape. In this case, as soon as the stress is removed, the nitinol will spontaneously return to its original shape. In this mode of use, nitinol behaves like a super spring, possessing an elastic range 10–30 times greater than that of a normal spring material. There are, however, constraints: the effect is only observed about 0–40 K above the Af temperature.
Nitinol is typically composed of approximately 50 to 51% nickel by atomic percent (55 to 56% weight percent).[9][10] Making small changes in the composition can change the transition temperature of the alloy significantly. One can control the Af temperature in nitinol to some extent, but convenient superelastic temperature ranges are from about −20 °C to +60 °C.
One often-encountered complication regarding nitinol is the so-called R-Phase. The R-Phase is another martensitic phase that competes with the martensite phase mentioned above. Because it does not offer the large memory effects of the martensite phase, it is, more often than not, an annoyance.
Manufacturing process
Nitinol is exceedingly difficult to make, due to the exceptionally tight compositional control required, and the tremendous reactivity of titanium. Every atom of titanium that combines with oxygen or carbon is an atom that is robbed from the NiTi lattice, thus shifting the composition and making the transformation temperature that much lower. There are two primary melting methods used today:
- Vacuum Arc Remelting: This is done by striking an electrical arc between the raw material and a water-cooled copper strike plate. Melting is done in a high vacuum, and the mold itself is water-cooled copper, so no carbon is introduced during melting.
- Vacuum Induction Melting: This is done by using alternating magnetic fields to heat the raw materials in a crucible (generally carbon). This is also done in a high vacuum, but carbon is introduced during the process.
While both methods have advantages, there are no substantive data showing that material from one process is better than the other. Other methods are also used on a boutique scale, including plasma arc melting, induction skull melting, and e-beam melting. Physical vapor deposition is also used on a laboratory scale.
Hot working of nitinol is relatively easy, but cold working is difficult because the enormous elasticity of the alloy increases die or roll contact, leading to tremendous frictional resistance and tool wear. For similar reasons, machining is extremely difficult—to make things worse, the thermal conductivity of nitinol is poor, so heat is difficult to remove. Grinding (abrasive cutting), Electrical discharge machining (EDM) and laser cutting are all relatively easy.
Heat treating nitinol is delicate and critical. It is the essential tool in fine-tuning the transformation temperature. Aging time and temperature controls the precipitation of various Ni-rich phases, and thus controls how much nickel resides on the NiTi lattice; by depleting the matrix of nickel, aging increases the transformation temperature. The combination of heat treatment and cold working is essential in controlling the properties of nitinol.[11]
Limitations
Fatigue failures of nitinol devices are a constant subject of discussion. Because it is the material of choice for applications requiring enormous flexibility and motion (e.g., peripheral stents and heart valves), it is necessarily exposed to much greater fatigue strains than are other metals. While the strain-controlled fatigue performance of nitinol is superior to all other known metals, fatigue failures have been observed in the most demanding applications. There is a great deal of effort underway trying to better understand and define the durability limits of nitinol.
Nitinol is half nickel, and thus there has been a great deal of concern in the medical industry regarding the release of nickel, a known allergen and possible carcinogen.[12] (Nickel is also present in substantial amounts in stainless steel and cobalt-chrome alloys.) When properly treated (via electropolishing and/or passivation), nitinol forms a very stable protective TiO2 layer that acts as a very effective and self-healing barrier against ion exchange. It has been repeatedly shown that nitinol releases nickel at a slower pace than stainless steel, for example. With that said, very early medical devices were made without electropolishing, and corrosion was observed. Today's nitinol vascular self-expandable metallic stents, for example, show no evidence of corrosion or nickel release, and the outcomes in patients with and without nickel allergies are indistinguishable.
There are constant and long-running discussions regarding inclusions in nitinol, both TiC and Ti2NiOx. All metals contain inclusions, and nitinol cannot be melted without inclusions—they are omnipresent. The size, distribution and type of inclusions can be controlled to some extent. Theoretically, smaller, rounder and few inclusions should lead to increased fatigue durability. All studies done to date, however, have failed to show measurable differences.[13][14]
A major limitation to further use of nitinol has been its difficulty to weld, both to itself and other materials. In the past ten years, laser welding nitinol to itself has become a relatively routine process. More recently, strong joints between NiTi wires and stainless steel wires have been made using nickel filler. More research is ongoing into other processes and other metals to which nitinol can be welded.[15]
Recent advances have shown that processing of Nitinol can expand thermomechanical capabilities, allowing for multiple shape memories to be embedded within a monolithic structure.[16] Research on multi-memory technology is on-going and promises to deliver enhanced shape memory devices in the near future.
Applications
There are four commonly used types of applications for nitinol.
- Free Recovery: nitinol is deformed at a low temperature, and heated to recover its original shape.
- Constrained Recovery: The same, except that recovery is rigidly prevented, and thus a stress is generated.
- Work Production: Here the alloy is allowed to recover, but to do so it must act against a force (thus doing work).
- Superelasticity: As discussed above, here the nitinol acts as a super spring.
In 1989 a survey was conducted in the United States and Canada that involved seven organizations. The survey focused on predicting the future technology, market, and applications of SMA's. The companies predicted the following uses of nitinol in a decreasing order of importance: (1) Couplings, (2) Biomedical and medical, (3) Toys, demonstration, novelty items, (4) Actuators, (5) Heat Engines, (6) Sensors, (7) Cryogenically activated die and bubble memory sockets, and finally (8) lifting devices.[17]
- Nitinol is also popular in extremely resilient glasses frames. It is also used in some mechanical watch springs.
- It can be used as a temperature control system; as it changes shape, it can activate a switch or a variable resistor to control the temperature.
- It is used in cell-phone technology as a retractable antenna, or microphone boom, due to its highly flexible and mechanical memory nature.
- It is used in some novelty products, such as self-bending spoons which can be used by amateur and stage magicians to demonstrate "psychic" powers or as a practical joke, as the spoon will bend itself when used to stir tea, coffee, or any other warm liquid.
- It can also be used as wires which are used to locate and mark breast tumours so that following surgery can be more exact.
- Due to the fact it can change shapes it is also used as a golf club insert.
- Nickel titanium can be used to make the underwires for underwire bras.[18][19][20]
Heat engines
- Demonstration model heat engines have been built which use nitinol wire to produce mechanical energy from hot and cold heat sources.[21] A prototype commercial engine developed in the 1970s by engineer Ridgway Banks at Lawrence Berkeley National Laboratory, was named the Banks Engine.[22][23][24][25][26]
Biocompatible applications
- Nitinol is highly biocompatible and has properties suitable for use in orthopaedic implants. Due to Nitinol's unique properties it has seen a large demand for use in less invasive medical devices. Nitinol tubing is commonly used in catheters, stents, and superelastic needles.
- In colorectal surgery , the material is used in devices for reconnecting the intestine after removing the pathology.
- In dentistry, the material is used in orthodontics for brackets and wires connecting the teeth. Suresmile is one example of an orthodontic application. Once the SMA is placed in the mouth its temperature rises to ambient body temperature. This causes the nitinol to contract back to its original shape, applying a constant force to move the teeth. These SMA wires do not need to be retightened as often as they can contract as the teeth move unlike conventional stainless steel wires. Additionally, nitinol can be used in endodontics, where nitinol files are used to clean and shape the root canals during the root canal procedure.
- Another significant application of nitinol in medicine is in stents: A collapsed stent can be inserted into a vein and heated (returning to its original expanded shape) helping to improve blood flow. Also, as a replacement for sutures where nitinol wire can be weaved through two structures then allowed to transform into its preformed shape which should hold the structures in place.
References
- ↑ W.J. Buehler, J.W. Gilfrich & R.C. Wiley, "Effects of low-temperature phase changes on the mechanical properties of alloys near composition TiNi," Journal of Applied Physics 34 (1963) p 475. doi:10.1063/1.1729603
- ↑ F.E. Wang, W.J. Buehler & S.J. Pickart, "Crystal structure and a unique martensitic transition of TiNi," Journal of Applied Physics 36 (1965) p 3232-3239.
- ↑ "The Alloy That Remembers", Time, 1968-09-13.
- ↑ Kauffman, George B.; Mayo, Isaac (1997), "The Story of Nitinol: The Serendipitous Discovery of the Memory Metal and Its Applications", The Chemical Educator 2: 1, doi:10.1007/s00897970111a.
- ↑ Olander, A (1932), J. Amer. Chem. Soc. 54: 3819
- ↑ Hornbogen, E. (1956), Z. Metallkunde 47: 47
- ↑ http://www.nitinol.com/nitinol-university/nitinol-facts/
- ↑ Funakubo, Hiroyasu (1984), Shape memory alloys, University of Tokyo, pp. 7, 176.
- ↑ 9.0 9.1 http://www.nitinol.com/media/files/material-properties-pdfs/sm495_wire_data%20%5BConverted%5D_v2.pdf
- ↑ http://www.nitinol.com/media/files/material-properties-pdfs/se508_wire_data%20%5BConverted%5D_v2.pdf
- ↑ Pelton, A.; Russell, S. and DiCello, J. (2003), "The physical metallurgy of nitinol for medical applications", JOM Journal of the Minerals, Metals and Materials Society 55 (5): 33
- ↑ http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s118nick.pdf
- ↑ Morgan, N.; A. Wick, J. DiCello, and R. Graham (2006), "Carbon and Oxygen Levels in Nitinol Alloys and the Implications for Medical Device Manufacture and Durability", Proceedings of the International Conference on Shape Memory and Superelastic Technologies (ASM International): 821
- ↑ Miyazaki, S.; Y Sugaya and K Otsuka (1989), MRS International Meeting On Advanced Materials (Materials Research Society) 9: 257
- ↑ Hall, Peter (2005), Method of Welding Titanium and Titanium Based Alloys to Ferrous Metals
- ↑ Khan, M. I.; Zhou Y. N. (2011), Methods and Systems for Processing Materials, Including Shape Memory Materials Unknown parameter
|note=ignored (help) - ↑ Miller, Richard K. and Terri Walker (1989), Survey on shape memory alloys, p. 17.
- ↑ Brady, George Stuart; Henry R. Clauser, John A. Vaccari (2002), Materials Handbook (15 ed.), McGraw-Hill Professional, p. 633, ISBN 978-0-07-136076-0, retrieved 2009-05-09
- ↑ Sang, David; Peter Ellis, Lawrie Ryan, Jane Taylor, Derek McMonagle, Louise Petheram, Phil Godding (2005), Scientifica (Illustrated ed.), Nelson Thornes, p. 80, ISBN 0-7487-7996-5, retrieved 2009-05-09
- ↑ Jones, Gail; Michael R. Falvo, Amy R. Taylor, Bethany P. Broadwell (2007), "Nanomaterials: Memory Wire", Nanoscale science (Illustrated ed.), NSTA Press, p. 109, ISBN 1-933531-05-3, retrieved 2009-05-09
- ↑ Nitinol Heat Engine Kit Images Scientific Instruments. 2007. Accessed 2011
- ↑ Ridgway Banks (July 7, 1975), Die Naturwissenschaften http://www.springerlink.com/content/nr31093w23446x0l/, retrieved 2012-11-05 Missing or empty
|title=(help) - ↑ Vimeo posting of "The Individualist", documentary on Ridgway Banks
- ↑ "Single wire nitinol engine", Ridgway M. Banks, US Patent
- ↑ "Metals that Remember", Popular Science, January 1988
- ↑ "Engine Uses No Fuel", Milwaukee Journal, December 5, 1973
Further reading
- H.R. Chen, ed., Shape Memory Alloys: Manufacture, Properties and Applications, Nova Science Publishers Inc., 2010, ISBN 978-1-60741-789-7.
- Y.Y. Chu & L.C. Zhao, eds., Shape Memory Materials and Its [sic] Applications, Trans Tech Publications Ltd., 2002, ISBN 0-87849-896-6.
- D.C. Lagoudas, ed., Shape Memory Alloys, Springer Science+Business Media LLC, 2008, ISBN 978-0-387-47684-1.
- K. Otsuka & C.M. Wayman, eds., Shape Memory Materials, Cambridge University Press, 1998, ISBN 0-521-44487-
- Sai V. Raj, Low Temperature Creep of Hot-extruded Near-stoichiometric NiTi Shape Memory Alloy, National Aeronautics and Space Administration, Glenn Research Center, 2013.
- Gerald Julien, Nitinol Technologies,Inc Edgewood, Wa. Us patent" 6422010 Manufacturing of Nitinol Parts & Forms
A process of making parts and forms of Type 60 Nitinol having a shape memory effect, comprising: selecting a Type 60 Nitinol. Inventor G,Julien CEO of Nitinol Technologies, Inc. (Washington State)
External links
| Wikimedia Commons has media related to Nickel titanium. |
- Society of Shape Memory and Superelastic Technologies
- Nitinol Resource Library
- Physical properties of nitinol
- Nitinol Technical Resource Library
- Literature on Nitinol Wire
- Nitinol-Tubing
Science Digest articles - Miracle Metal 1982 - PDF