Negishi coupling
The Negishi coupling is a cross coupling reaction in organic chemistry involving an organozinc compound, an organic halide and a nickel or palladium catalyst creating a new carbon–carbon covalent bond:[1][2]
- The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions.
- The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl.
- The halide X' in the organozinc compound can be chloride, bromine or iodine and the organic residue R' is alkenyl, aryl, allyl or alkyl.
- The metal M in the catalyst is nickel or palladium
- The ligand L in the catalyst can be triphenylphosphine, dppe, BINAP or chiraphos
Palladium catalysts in general have higher chemical yields and higher functional group tolerance.
The reaction is named after Ei-ichi Negishi who received the 2010 Nobel Prize in Chemistry for the discovery and development of this reaction.
Reaction mechanism
The active catalyst in this reaction is zerovalent (M0) and the reaction in general proceeds through an oxidative addition step of the organic halide followed by transmetalation with the zinc compound and then reductive elimination:
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R' resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.[3]
A common side reaction is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide:[4]
- Ar–Pd–Ar' + Ar'–Zn–X → Ar'–Pd–Ar' + Ar–Zn–X
- Ar'–Pd–Ar' → Ar'–Ar' + Pd(0) (homocoupling)
- Ar–Zn–X + H2O → Ar–H + HO–Zn–X (reaction accompanied by dehalogenation)
Scope
The Negishi coupling has been applied in the synthesis of a 2,2'-bipyridine from 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0),[5] the synthesis of a biphenyl from o-tolylzinc chloride and o-iodotoluene and tetrakis(triphenylphosphine)palladium(0),[6] the synthesis of 5,7-hexadecadiene from 1-decyne and (Z)-1-hexenyl iodide.[7]

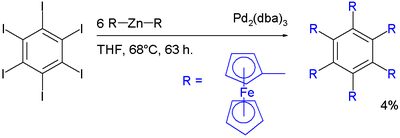
with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0) in tetrahydrofuran. The yield is only 4% signifying substantial crowding around the aryl core.
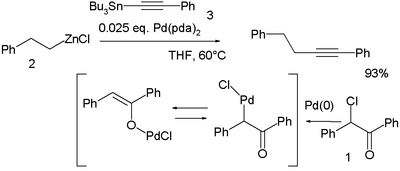
In a novel modification palladium is first oxidized by the haloketone 2-chloro-2-phenylacetophenone 1 and the resulting palladium OPdCl complex then accepts both the organozinc compound 2 and the organotin compound 3 in a double transmetalation:[9]
See also
External links
- The Negishi coupling at www.organic-chemistry.org: Link
- Negishi coupling – synthetic protocols from organic-reaction.com
References
- ↑ Anthony O. King, Nobuhisa Okukado and Ei-ichi Negishi (1977). "Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides". Journal of the Chemical Society Chemical Communications (19): 683. doi:10.1039/C39770000683.
- ↑ Strategic Applications of Named Reactions in Organic Synthesis Laszlo Kurti, Barbara Czako Academic Press (March 4, 2005) ISBN 0-12-429785-4
- ↑ Juan A. Casares, Pablo Espinet, Beatriz Fuentes, and Gorka Salas (2007). "Insights into the Mechanism of the Negishi Reaction: ZnRX versus ZnR2 Reagents". J. Amer. Chem. Soc. 129 (12): 3508. doi:10.1021/ja070235b. PMID 17328551.
- ↑ Revealing a Second Transmetalation Step in the Negishi Coupling and Its Competition with Reductive Elimination: Improvement in the Interpretation of the Mechanism of Biaryl Syntheses Qiang Liu, Yu Lan, Jing Liu, Gang Li, Yun-Dong Wu and Aiwen Lei J. Am. Chem. Soc., Article ASAP doi:10.1021/ja903277d
- ↑ Adam P. Smith, Scott A. Savage, J. Christopher Love, and Cassandra L. Fraser (2004), "Synthesis of 4-, 5-, and 6-methyl-2,2'-bipyridine by a Negishi cross-coupling strategy: 5-methyl-2,2'-bipyridine", Org. Synth.; Coll. Vol. 10: 517
- ↑ Ei-ichi Negishi, Tamotsu Takahashi, and Anthony O. King (1993), "Synthesis of biaryls via palladium-catalyzed cross-coupling: 2-methyl-4'-nitrobiphenyl", Org. Synth.; Coll. Vol. 8: 430
- ↑ Ei-ichi Negishi, Tamotsu Takahashi, and Shigeru Baba (1993), "Palladium-catalyzed synthesis of conjugated dienes", Org. Synth.; Coll. Vol. 8: 295
- ↑ Yong Yu, Andrew D. Bond, Philip W. Leonard, Ulrich J. Lorenz, Tatiana V. Timofeeva, K. Peter C. Vollhardt, Glenn D. Whitener and Andrey A. Yakovenko (2006). "Hexaferrocenylbenzene". Chem. Commun. (24): 2572–2574. doi:10.1039/b604844g. PMID 16779481.
- ↑ Yingsheng Zhao, Haibo Wang, Xiaohui Hou, Yanhe Hu, Aiwen Lei, Heng Zhang, and Lizheng Zhu (2006). "Oxidative Cross-Coupling through Double Transmetallation: Surprisingly High Selectivity for Palladium-Catalyzed Cross-Coupling of Alkylzinc and Alkynylstannanes". J. Amer. Chem. Soc. 128 (47): 15048. doi:10.1021/ja0647351. PMID 17117830.