Nedocromil
 | |
|---|---|
| Systematic (IUPAC) name | |
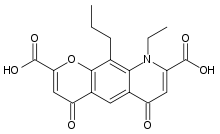
| 9-ethyl-4,6-dioxo-10-propyl-6,9-dihydro-4H-pyrano[3,2-g]quinoline-2,8-dicarboxylic acid | |
| Clinical data | |
| Trade names | Alocril |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601243 |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Inhalation and eye drops |
| Pharmacokinetic data | |
| Protein binding | 89% |
| Metabolism | not metabolized |
| Half-life | ~3.3 hours |
| Excretion | excreted unchanged |
| Identifiers | |
| CAS number | 69049-73-6 |
| ATC code | R01AC07 R03BC03, S01GX04 |
| PubChem | CID 50294 |
| DrugBank | DB00716 |
| ChemSpider | 45608 |
| UNII | 0B535E0BN0 |
| KEGG | D05129 |
| ChEBI | CHEBI:7492 |
| ChEMBL | CHEMBL746 |
| Chemical data | |
| Formula | C19H17NO7 |
| Mol. mass | 371.341 g/mol |
| SMILES
| |
| |
| | |
Nedocromil sodium is a medication used to prevent wheezing, shortness of breath, and other breathing problems caused by asthma. It is administered by an inhaler under the brand name Tilade (although its effects in this form are far less than those in albuterol or other well-known inhaler medications) and as an eye drop under the brand name Alocril.[1] [2] Liquid preparations are available in the UK under the name Rapitil for use for allergic eye reactions.[3]
Nedocromil is classified as a cromone. Nedocromil acts as a mast cell stabilizer, inhibits the degranulation of mast cells, prevents release of histamine and tryptase, so preventing the synthesis of prostaglandins and leukotrienes. US Production of inhaled nedocromil ceased in April 2008.[4]
References
- ↑ "ALOCRIL Product Information". ALLERGAN. Retrieved 17 May 2013.
- ↑ "ALOCRIL (nedocromil sodium) solution/ drops". NIH. Retrieved 17 May 2013.
- ↑ "Nedocromil eye drops" (in English). 2007-02-02. Retrieved 2009-08-04.
- ↑ http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/ucm089433.pdf
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||