Natural rubber

Natural rubber, also called India rubber or caoutchouc, as initially produced, consists of suitable polymers of the organic compound isoprene, with minor impurities of other organic compounds plus water. Forms of polyisoprene that are useful as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from certain trees. The latex is a sticky, milky colloid drawn off by making incisions into the bark and collecting the fluid in vessels in a process called "tapping". The latex then is refined into rubber ready for commercial processing. Natural rubber is used extensively in many applications and products, either alone or in combination with other materials. In most of its useful forms, it has a large stretch ratio, high resilience, and is extremely waterproof.[1]
Varieties
The major commercial source of natural rubber latex is the Pará rubber tree (Hevea brasiliensis), a member of the spurge family, Euphorbiaceae. This species is widely used because it grows well under cultivation and a properly managed tree responds to wounding by producing more latex for several years.
Many other plants produce forms of latex rich in isoprene polymers, though not all produce usable forms of polymer as easily as the Pará rubber tree does; some of them require more elaborate processing to produce anything like usable rubber, and most are more difficult to tap. Some produce other desirable materials, for example gutta-percha (Palaquium gutta)[2] and chicle from Manilkara species. Others that have been commercially exploited, or at least have shown promise as sources of rubber, include the rubber fig (Ficus elastica), Panama rubber tree (Castilla elastica), various spurges (Euphorbia spp.), lettuce (Lactuca species), the related Scorzonera tau-saghyz, various Taraxacum species, including common dandelion (Taraxacum officinale) and Russian dandelion (Taraxacum kok-saghyz), and guayule (Parthenium argentatum). The term gum rubber is sometimes applied to the tree-obtained version of natural rubber in order to distinguish it from the synthetic version.[1]
Discovery of commercial potential
The Para rubber tree is indigenous to South America. Charles Marie de La Condamine is credited with introducing samples of rubber to the Académie Royale des Sciences of France in 1736.[3] In 1751, he presented a paper by François Fresneau to the Académie (eventually published in 1755) which described many of the properties of rubber. This has been referred to as the first scientific paper on rubber.[3] In England, Joseph Priestley, in 1770, observed that a piece of the material was extremely good for rubbing off pencil marks on paper, hence the name "rubber". Later, it slowly made its way around England.
South America remained the main source of the limited amounts of latex rubber used during much of the 19th century. The trade was well protected and exporting seeds from Brazil was said to be a capital offense, although there was no law against it. Nevertheless, in 1876, Henry Wickham smuggled 70,000 Para rubber tree seeds from Brazil and delivered them to Kew Gardens, England. Only 2,400 of these germinated after which the seedlings were then sent to India, Ceylon (Sri Lanka), Indonesia, Singapore, and British Malaya. Malaya (now Malaysia) was later to become the biggest producer of rubber. In the early 1900s, the Congo Free State in Africa was also a significant source of natural rubber latex, mostly gathered by forced labor. Liberia and Nigeria also started production of rubber.
In India, commercial cultivation of natural rubber was introduced by the British planters, although the experimental efforts to grow rubber on a commercial scale in India were initiated as early as 1873 at the Botanical Gardens, Calcutta. The first commercial Hevea plantations in India were established at Thattekadu in Kerala in 1902.
In Singapore and Malaya, commercial production of rubber was heavily promoted by Sir Henry Nicholas Ridley, who served as the first Scientific Director of the Singapore Botanic Gardens from 1888 to 1911. He distributed rubber seeds to many planters and developed the first technique for tapping trees for latex without causing serious harm to the tree.[4] Because of his very fervent promotion of this crop, he is popularly remembered by the nickname "Mad Ridley".[5]
Properties

Rubber exhibits unique physical and chemical properties. Rubber's stress-strain behavior exhibits the Mullins effect and the Payne effect, and is often modeled as hyperelastic. Rubber strain crystallizes.
Owing to the presence of a double bond in each repeat unit, natural rubber is susceptible to vulcanisation and sensitive to ozone cracking.
The two main solvents for rubber are turpentine and naphtha (petroleum). The former has been in use since 1764 when François Fresnau made the discovery. Giovanni Fabbroni is credited with the discovery of naphtha as a rubber solvent in 1779. Because rubber does not dissolve easily, the material is finely divided by shredding prior to its immersion.
An ammonia solution can be used to prevent the coagulation of raw latex while it is being transported from its collection site.
Elasticity
On a microscopic scale, relaxed rubber is a disorganized cluster of erratically changing wrinkled chains. In stretched rubber, the chains are almost linear. The restoring force is due to the preponderance of wrinkled conformations over more linear ones. For the quantitative treatment see Ideal chain, for more examples see Entropic force.
Cooling below the glass transition temperature still permits local conformational changes but a reordering is practically impossible because of the larger energy barrier for the concerted movement of longer chains. "Frozen" rubber's elasticity is low and strain results from small changes of bond lengths and angles. (This caused the Challenger disaster, where flattened o-rings failed to relax to fill a widening gap.) The glass transition is fast and reversible: the force resumes on heating.
The parallel chains of stretched rubber are susceptible to crystallization. This takes some time because turns of twisted chains have to move out of the way of the growing crystallites. Crystallization has occurred, for example, when, after days, an inflated toy balloon is found withered at a relatively large remaining volume. Where it is touched, it shrinks because the temperature of the hand is enough to melt the crystals.
Vulcanization of rubber creates disulfide bonds between chains, which limits the degrees of freedom and results in chains that tighten more quickly for a given strain, thereby increasing the elastic force constant and making the rubber harder and less extensible.
Chemical makeup
Latex is the polymer cis-1,4-polyisoprene – with a molecular weight of 100,000 to 1,000,000 daltons. Typically, a small percentage (up to 5% of dry mass) of other materials, such as proteins, fatty acids, resins, and inorganic materials (salts) are found in natural rubber. Polyisoprene can also be created synthetically, producing what is sometimes referred to as "synthetic natural rubber", but the synthetic and natural routes are completely different.[1]
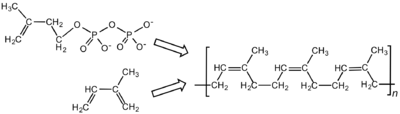
Some natural rubber sources, such as gutta-percha, are composed of trans-1,4-polyisoprene, a structural isomer that has similar, but not identical, properties.
Natural rubber is an elastomer and a thermoplastic. Once the rubber is vulcanized, it will turn into a thermoset. Most rubber in everyday use is vulcanized to a point where it shares properties of both; i.e., if it is heated and cooled, it is degraded but not destroyed.
The final properties of a rubber item depend not just on the polymer, but also on modifiers and fillers, such as carbon black, factice, whiting, and a host of others.
Biosynthesis
Rubber particles are formed in the cytoplasm of specialized latex-producing cells called laticifers within rubber plants.[6] Rubber particles are surrounded by a single phospholipid membrane with hydrophobic tails pointed inward. The membrane allows biosynthetic proteins to be sequestered at the surface of the growing rubber particle, which allows new monomeric units to be added from outside the biomembrane, but within the lacticifer. The rubber particle is an enzymatically active entity that contains three layers of material, the rubber particle, a biomembrane, and free monomeric units. The biomembrane is held tightly to the rubber core due to the high negative charge along the double bonds of the rubber polymer backbone.[7] Free monomeric units and conjugated proteins make up the outer layer. The rubber precursor is isopentenyl pyrophosphate (an allylic compound), which elongates by Mg2+-dependent condensation by the action of rubber transferase. The monomer adds to the pyrophosphate end of the growing polymer.[8] The process displaces the terminal high-energy pyrophosphate. The reaction produces a cis polymer. The initiation step is catalyzed by prenyltransferase, which converts three monomers of isopentenyl pyrophosphate into farnesyl pyrophosphate.[9] The farnesyl pyrophosphate can bind to rubber transferase to elongate a new rubber polymer.
The required isopentenyl pyrophosphate is obtained from the mevalonate pathway, which is derives from acetyl-CoA in the cytosol. In plants, isoprene pyrophosphate can also be obtained from 1-deox-D-xyulose-5-phosphate/2-C-methyl-D-erythritol-4-phosphate pathway within plasmids.[10] The relative ratio of the farnesyl pyrophosphate initiator unit and isoprenyl pyrophosphate elongation monomer determines the rate of new particle synthesis versus elongation of existing particles. Though rubber is known to be produced by only one enzyme, extracts of latex have shown numerous small molecular weight proteins with unknown function. The proteins possibly serve as cofactors, as the synthetic rate decreases with complete removal.[11]
Current sources
Close to 21 million tons of rubber were produced in 2005, of which approximately 42% was natural. Since the bulk of the rubber produced is of the synthetic variety, which is derived from petroleum, the price of natural rubber is determined, to a large extent, by the prevailing global price of crude oil.[12][13] Today, Asia is the main source of natural rubber, accounting for about 94% of output in 2005. The three largest producing countries, Thailand, Indonesia (2.4m tons)[14] and Malaysia, together account for around 72% of all natural rubber production. Natural rubber is not cultivated widely in its native continent of South America due to the existence of South American leaf blight, and other natural predators of the rubber tree.
Cultivation

Rubber latex is extracted from rubber trees. The economic life period of rubber trees in plantations is around 32 years – up to 7 years of immature phase and about 25 years of productive phase.
The soil requirement of the plant is generally well-drained, weathered soil consisting of laterite, lateritic types, sedimentary types, nonlateritic red, or alluvial soils.
The climatic conditions for optimum growth of rubber trees are:
- Rainfall of around 250 cm evenly distributed without any marked dry season and with at least 100 rainy days per year
- Temperature range of about 20 to 34°C, with a monthly mean of 25 to 28°C
- High atmospheric humidity of around 80%
- Bright sunshine amounting to about 2000 hours per year at the rate of six hours per day throughout the year
- Absence of strong winds
Many high-yielding clones have been developed for commercial planting. These clones yield more than 2,000 kg of dry rubber per hectare per year, when grown under ideal conditions.
Collection

In places such as Kerala, where coconuts are in abundance, the half shell of coconut is used as the collection container for the latex, but glazed pottery or aluminium or plastic cups are more common elsewhere. The cups are supported by a wire that encircles the tree. This wire incorporates a spring so it can stretch as the tree grows. The latex is led into the cup by a galvanised "spout" knocked into the bark. Tapping normally takes place early in the morning, when the internal pressure of the tree is highest. A good tapper can tap a tree every 20 seconds on a standard half-spiral system, and a common daily "task" size is between 450 and 650 trees. Trees are usually tapped on alternate or third days, although many variations in timing, length, and number of cuts are used. The latex, which contains 25–40% dry rubber, is in the bark, so the tapper must avoid cutting right through to the wood, else the growing cambial layer will be damaged and the renewing bark will be badly deformed, making later tapping difficult. It is usual to tap a pannel at least twice, sometimes three times, during the tree's life. The economic life of the tree depends on how well the tapping is carried out, as the critical factor is bark consumption. A standard in Malaysia for alternate daily tapping is 25 cm (vertical) bark consumption per year. The latex tubes in the bark ascend in a spiral to the right. For this reason, tapping cuts usually ascend to the left to cut more tubes.
The trees will drip latex for about four hours, stopping as latex coagulates naturally on the tapping cut, thus blocking the latex tubes in the bark. Tappers usually rest and have a meal after finishing their tapping work, then start collecting the liquid "field latex" at about midday. Some trees will continue to drip after the collection and this leads to a small amount of "cup lump" which is collected at the next tapping. The latex that coagulates on the cut is also collected as "tree lace". Tree lace and cup lump together account for 10–20% of the dry rubber produced. Latex that drips onto the ground, "earth scrap", is also collected periodically for processing of low-grade product.
Field coagula


There are four types of field coagula, "cuplump", "treelace", "smallholders’ lump" and "earth scrap". Each has significantly different properties.[15]
Cuplump is the coagulated material found in the collection cup when the tapper next visits the tree to tap it again. It arises from latex clinging to the walls of the cup after the latex was last poured into the bucket, and from late-dripping latex exuded before the latex-carrying vessels of the tree become blocked. It is of higher purity and of greater value than the other three types.
Treelace is the coagulum strip that the tapper peels off the previous cut before making a new cut. It usually has higher copper and manganese contents than cuplump. Both copper and manganese are pro-oxidants and can lower the physical properties of the dry rubber.
Smallholders’ lump is produced by smallholders who collect rubber from trees a long way away from the nearest factory. Many Indonesian smallholders, who grow paddy in remote areas, tap dispersed trees on their way to work in the paddy fields and collect the latex (or the coagulated latex) on their way home. As it is often impossible to preserve the latex sufficiently to get it to a factory that processes latex in time for it to be used to make high quality products, and as the latex would anyway have coagulated by the time it reached the factory, the smallholder will coagulate it by any means available, in any container available. Some smallholders use small containers, buckets etc., but often the latex is coagulated in holes in the ground, which are usually (but not always) lined with plastic. Acidic materials and fermented fruit juices are used to coagulate the latex – a form of assisted biological coagulation. Little care is taken to exclude twigs, leaves, and even bark from the lumps that are formed, which may also include treelace collected by the smallholder.
Earth scrap is the material that gathers around the base of the tree. It arises from latex overflowing from the cut and running down the bark of the tree, from rain flooding a collection cup containing latex, and from spillage from tappers’ buckets during collection. It contains soil and other contaminants, and has variable rubber content depending on the amount of contaminants mixed with it. Earth scrap is collected by the field workers two or three times a year and may be cleaned in a scrap-washer to recover the rubber, or sold off to a contractor who will clean it and recover the rubber. It is of very low quality and under no circumstances should it be included in block rubber or brown crepe.
Processing

The latex will coagulate in the cups if kept for long. The latex has to be collected before coagulation. The collected latex, "field latex", is transferred into coagulation tanks for the preparation of dry rubber or transferred into air-tight containers with sieving for ammoniation. Ammoniation is necessary to preserve the latex in a colloidal state for longer periods of time.
Latex is generally processed into either latex concentrate for manufacture of dipped goods or it can be coagulated under controlled, clean conditions using formic acid. The coagulated latex can then be processed into the higher-grade, technically specified block rubbers such as SVR 3L or SVR CV or used to produce Ribbed Smoke Sheet grades.
Naturally coagulated rubber (cup lump) is used in the manufacture of TSR10 and TSR20 grade rubbers. The processing of the rubber for these grades is a size reduction and cleaning process to remove contamination and prepare the material for the final stage of drying.[16]
The dried material is then baled and palletized for storage and shipment in various methods of transportation.
Transportation
Natural rubber latex is shipped from factories in south-west Asia, South America, and North Africa to destinations around the world. As the cost of natural rubber has risen significantly, the shipping methods which offer the lowest cost per unit of weight are preferred. Depending on the destination, warehouse availability, and transportation conditions, some methods are more suitable to certain buyers than others. In international trade, latex rubber is mostly shipped in 20-foot ocean containers. Inside the ocean container, various types of smaller containers are used by factories to store latex rubber.[17]
Uses

Contemporary manufacturing
Around 25 million tonnes of rubber is produced each year, of which 42 percent is natural rubber. The remainder is synthetic rubber derived from petrochemical sources. Around 70 percent of the world's natural rubber is used in tires. The top end of latex production results in latex products such as surgeons' gloves, condoms, balloons and other relatively high-value products. The mid-range which comes from the technically-specified natural rubber materials ends up largely in tires but also in conveyor belts, marine products, windshield wipers and miscellaneous rubber goods. Natural rubber offers good elasticity, while synthetic materials tend to offer better resistance to environmental factors such as oils, temperature, chemicals or ultraviolet light and suchlike. "Cured rubber" is rubber which has been compounded and subjected to the vulcanisation process which creates cross-links within the rubber matrix.
Prehistoric uses
The first use of rubber was by the Olmecs, who centuries later passed on the knowledge of natural latex from the Hevea tree in 1600 BC to the ancient Mayans. They boiled the harvested latex to make a ball for a Mesoamerican ballgame.[18]
Pre-World War II manufacturing
Other significant uses of rubber are door and window profiles, hoses, belts, gaskets, matting, flooring, and dampeners (antivibration mounts) for the automotive industry. Gloves (medical, household and industrial) and toy balloons are also large consumers of rubber, although the type of rubber used is concentrated latex. Significant tonnage of rubber is used as adhesives in many manufacturing industries and products, although the two most noticeable are the paper and the carpet industries. Rubber is also commonly used to make rubber bands and pencil erasers.
Pre-World War II textile applications
Rubber produced as a fiber, sometimes called 'elastic', has significant value for use in the textile industry because of its excellent elongation and recovery properties. For these purposes, manufactured rubber fiber is made as either an extruded round fiber or rectangular fibers that are cut into strips from extruded film. Because of its low dye acceptance, feel and appearance, the rubber fiber is either covered by yarn of another fiber or directly woven with other yarns into the fabric. In the early 1900s, for example, rubber yarns were used in foundation garments. While rubber is still used in textile manufacturing, its low tenacity limits its use in lightweight garments because latex lacks resistance to oxidizing agents and is damaged by aging, sunlight, oil, and perspiration. Seeking a way to address these shortcomings, the textile industry has turned to neoprene (polymer of chloroprene), a type of synthetic rubber, as well as another more commonly used elastomer fiber, spandex (also known as elastane), because of their superiority to rubber in both strength and durability.
Vulcanization
Natural rubber is often vulcanized, a process by which the rubber is heated and sulfur, peroxide or bisphenol are added to improve resistance and elasticity, and to prevent it from perishing. The development of vulcanization is most closely associated with Charles Goodyear in 1839.[19] Before World War II era manufacturing, carbon black was often used as an additive to rubber to improve its strength, especially in vehicle tires.
Allergic reactions
Some people have a serious latex allergy, and exposure to natural latex rubber products such as latex gloves can cause anaphylactic shock. The antigenic proteins found in Hevea latex may be deliberately reduced (though not eliminated)[20] through processing.
Latex from non-Hevea sources, such as Guayule, can be used without allergic reaction by persons with an allergy to Hevea latex.[21]
Some allergic reactions are not to the latex itself, but from residues of chemicals used to accelerate the cross-linking process. Although this may be confused with an allergy to latex, it is distinct from it, typically taking the form of Type IV hypersensitivity in the presence of traces of specific processing chemicals.[20][22]
Alternative sources
Dandelion milk had been known to contain latex for a long time. The latex exhibited the same quality as the natural rubber from rubber trees. Yet in the wild types of dandelion, the latex content is low and varies greatly. By inhibiting one key enzyme and using modern cultivation methods and optimization techniques, scientists in the Fraunhofer Institute for Molecular Biology and Applied Ecology (IME) in Germany developed a cultivar that is suitable for commercial production of natural rubber.[23] In collaboration with Continental Tires, IME is building a pilot facility. The first prototype test tires made with blends from dandelion-rubber are scheduled to be tested on public roads over the next few years.
Microbial degradation
Natural rubber is susceptible to degradation by a wide range of bacteria.[24][25][26][27][28][29][30][31]
See also
- Akron, Ohio, center of the rubber industry in the USA
- Condoms, also called "rubbers"
- Crepe rubber
- Ebonite
- Emulsion dispersion
- Fordlândia, failed attempt to establish a rubber plantation in Brazil
- Reinforced rubber
- Resilin, a rubber substitute
- Rubber seed oil
- Rubber technology
- Stevenson Plan, historical British plan to stabilize rubber prices
- Charles Greville Williams, researched natural rubber being a polymer of the monomer isoprene
References
Notes
- ↑ 1.0 1.1 1.2 Heinz-Hermann Greve "Rubber, 2. Natural" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_225
- ↑ Burns, Bill. "The Gutta Percha Company". History of the Atlantic Cable & Undersea Communications. Retrieved 14 February 2009.
- ↑ 3.0 3.1 http://www.bouncing-balls.com/timeline/people/nr_condamine.htm
- ↑ Cornelius-Takahama, Vernon (2001). "Sir Henry Nicholas Ridley". Singapore Infopedia. Retrieved 9 February 2013.
- ↑ Dr Loh Wei Leng and Khor Jin Keong (19 September 2011). "Mad Ridley and the rubber boom". Malaysia History. Retrieved 9 February 2013.
- ↑ N. Ohya; T. Koyama, (2001). “Biosynthesis of Natural Rubber and Other Natural Polyisoprenoids”. Biopolymers Polyisoprenoids. 2 73-81. ISBN 978-3-527-30221-5
- ↑ J.C. Paterson-Jones, M.G. Gilliland, J. Van Staden, The Biosynthesis of Natural Rubber, Journal of Plant Physiology, Volume 136, Issue 3, June 1990, Pages 257-263, ISSN 0176-1617, 10.1016/S0176-1617(11)80047-7.
- ↑ Natural Rubber Biosynthesis and Physics - Chemical Studies on Plant Derived Latex, Biotechnology of Biopolymers - Magdy Elnashar (Ed.). 2011. ISBN 978-953-307-179-4.
- ↑ W. Xie; C. M. McMahan; A.J. DeGraw’ M. D. Distefano; K. Cornish; M. C. Whalen; D. K. Shintani, “Initiation of rubber synthesis: In vitro comparisons of benzophenone-modified diphosphate analogues in three rubber preducing species”, Phytochemistry 69 (2008) 2539–2545
- ↑ P. J. Casey; M. C. Seabra, (1996). "Protein Prenyltransferases". Journal of Biological Chemistry 271 (10): 5289–5292.
- ↑ H. Kang; M. Y. Kang; K. H. Han, “Identification of Natural Rubber and Characterization of Biosynthetic Activity”,Plant Physiol. 2000 July; 123 (3), 1133-1142.
- ↑ "Overview of the Causes of Natural Rubber Price Volatility". En.wlxrubber.com. 2010-02-01. Retrieved 2013-03-21.
- ↑ Short run and long run effects of the world crude oil prices on the Malaysian natural rubber and palm oil export prices
- ↑ Listiyorini, Eko (2010-12-16). "bloomberg.com". bloomberg.com. Retrieved 2013-03-21.
- ↑ This section has been copied almost verbatim from the public domain UN Food and Agriculture Organization (FAO), ecoport.com article: Cecil, John; Mitchell, Peter; Diemer, Per; Griffee, Peter (2013). "Processing of Natural Rubber, Manufacture of Latex-Grade Crepe Rubber". ecoport.org. FAO, Agricultural and Food Engineering Technologies Service. Retrieved March 19, 2013.
- ↑ Technical Grades and Basis for Grading by ASTM D2227 - Basic Rubber Testing
- ↑ Transportation of Natural Rubber - Industry Source
- ↑ "The Mayan-Olmec Connection". Maya12-21-2012.com. 2012-12-21. Retrieved 2013-03-21.
- ↑ Slack, Charles. "Noble Obsession: Charles Goodyear, Thomas Hancock, and the Race to Unlock the Greatest Industrial Secret of the Nineteenth Century". Hyperion 2002. [ISBN 9780786867899]
- ↑ 20.0 20.1 "Premarket Notification [510(k)] Submissions for Testing for Skin Sensitization To Chemicals In Natural Rubber Products". FDA. Retrieved 22 September 2013.
- ↑ http://www.fda.gov/forconsumers/consumerupdates/ucm048052.htm
- ↑ American Latex Allergy Association. "Allergy Fact Sheet".
- ↑ "Making Rubber from Dandelion Juice". sciencedaily.com. sciencedaily.com. Retrieved 22 November 2013.
- ↑ Rook, J.J. (1955) Microbiological deterioration of vulcanized rubber. Appl. Microbiol. 3, 302-309.
- ↑ Lee£ang, K.W.H. (1963) Microbiologic degradation of rubber. J. Am. Water Works Assoc. 53, 1523-1535.
- ↑ Tsuchii, A., Suzuki, T. and Takeda, K. (1985) Microbial degradation of natural rubber vulcanizates. Appl. Environ. Microbiol. 50, 965-970.
- ↑ Heisey, R.M. and Papadatos, S. (1995) Isolation of microorganisms able to metabolize puri¢ed natural rubber. Appl. Environ. Microbiol. 61, 3092-3097.
- ↑ Jendrossek, D., Tomasi, G. and Kroppenstedt, R.M. (1997) Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiol. Lett. 150, 179-188.
- ↑ Linos, A. and Steinbu« chel, A. (1998) Microbial degradation of natural and synthetic rubbers by novel bacteria belonging to the genus Gordona. Kautsch. Gummi Kunstst. 51, 496-499.
- ↑ Linos, A., Steinbu« chel, A., Spro« er, C. and Kroppenstedt, R.M. (1999) Gordonia polyisoprenivorans sp. nov., a rubber degrading actinomycete isolated from automobile tire. Int. J. Syst. Bacteriol. 49, 1785-1791.
- ↑ Alexandros Linos; Rudolf Reichelt; Ulrike Keller; Alexander Steinbuchel (October 199). "A Gram-negative bacterium, identified as Pseudomonas aeruginosa AL98, is a potent degrader of natural rubber and synthetic cis-1,4-polyisoprene". FEMS Microbiology Letter.
Bibliography
- Ascherson, Neal. (1963). The King Incorporated. Allen & Unwin. ISBN 1-86207-290-6 (1999 Granta edition).
- Brydson, J.A. Rubbery Materials and their Compounds
- Hobhouse, Henry (2005 [2003]). Seeds of Wealth: Five Plants That Made Men Rich. Shoemaker & Hoard. pp. 125–185. ISBN 1-59376-089-2.
- Hochschild, Adam. (1998). King Leopold’s Ghost: A Story of Greed, Terror, and Heroism in Colonial Africa. Mariner Books. ISBN 0-330-49233-0.
- Morton, Maurice. Rubber Technology
- Petringa, Maria. (2006). Brazza, A Life for Africa. Bloomington, IN: AuthorHouse. ISBN 978-1-4259-1198-0
External links
| Look up natural rubber in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Rubber. |
History
Associations
- International Rubber Research and Development Board (IRRDB)
- Association of Natural Rubber Producing Countries (ANRPC)
- International Rubber Study Group (IRSG)
- Associação Paulista de Produtores e Beneficiadores de Borracha (APABOR), Brazil (Portuguese)
- Malaysian Rubber Board (LGM)
- Thailand Rubber Association (TRA)
- Rubber Research Institute of Sri Lanka
Other
- Database for natural rubber, by National Laboratories of Medicine
- Rubber-Chemical Compatibility Guide
- Basic Rubber Testing
- Rubber-Products and Prices
| |||||||||||||||||||||||||||||||||||

