Naphthenic acid
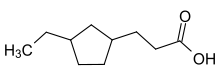
Naphthenic acid (CAS: 1338-24-5 ) is the name for an unspecific mixture of several cyclopentyl and cyclohexyl carboxylic acids with molecular weight of 120 to well over 700 atomic mass units. The main fraction are carboxylic acids with a carbon backbone of 9 to 20 carbons. Salts of naphthenic acids, which are naphthenates, are widely used as hydrophobic sources of metal ions in diverse applications.[1]
Preparation and occurrence
They are obtained by oxidation of the naphtha fraction of the crude oil refining. The composition varies with the crude oil composition and the conditions during refining and oxidation.[2] Naphthenic acids are present in crude oil and leads to corrosion problems within the oil refineries, therefore "naphthenic acid corrosion" phenomena are well researched.[3][4] Crude oils with a high content of naphthenic acids are often referred to as high total acid number (TAN) crude oils or high acid crude oil (HAC). Naphthenic acids are the major contaminant in water used for extraction of oil from tar sands.[5] Naphthenic acids have both acute and chronic toxicity to fish and other organisms.[5]
Metal naphthenates
Naphthenates are the salts of naphthenic acids. Analogous to the corresponding acetate, which are better defined but less useful, metal naphthenates are coordination complexes. They have the formula M(naphthenate)2 or are basic oxides with the formula M3O(naphthenate)6. The naphthenates are highly soluble in organic media, such as paints. They have industrial applications including synthetic detergents, lubricants, corrosion inhibitors, fuel and lubricating oil additives, wood preservatives, insecticides, fungicides, acaricides, wetting agents, and oil drying agents used in painting and wood surface treatment. Industrially useful naphthenates include those of magnesium, calcium, barium, cobalt, copper, lead, manganese, nickel, vandyl, and zinc.[1]
See also
References
- ↑ 1.0 1.1 Angelo Nora, Alfred Szczepanek, Gunther Koenen, "Metallic Soaps" in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_361
- ↑ Walter E. Rudzinski, Leon Oehlers, and Yi Zhang (2002). "Tandem Mass Spectrometric Characterization of Commercial Naphthenic Acids and a Maya Crude Oil". Energy Fuels 16 (5): 1178–1185. doi:10.1021/ef020013t.
- ↑ Slavcheva E.; Shone B.; Turnbull A. (1999). "Review of naphthenic acid corrosion in oilrefining". British Corrosion Journal 34 (2): 125–131. doi:10.1179/000705999101500761.
- ↑ "Article with details concerning naphthenic acid corrosion".
- ↑ 5.0 5.1 Allen, E. W. (2008). "Process water treatment in Canada’s oil sands industry: I. Target pollutants and treatment objectives". Journal of Environmental Engineering and Science 7 (2): 123–138. doi:10.1139/S07-038.
External links
- Article concerning refining crude oil with a high content of naphthenic acids
- Crude oils with a high content of naphthenic acids in China's refineries
- Crude oils containing naphthenic acids in the Grangemouth refinery
- Overview of naphthenic acid corrosion
- Literature survey of naphthenic acid corrosion
- Removing naphthenic acids from the crude oil
- Presentation by Nalco on naphthenic acid corrosion
- Presentation by Baker Petrolite on naphthenic acid corrosion
- Presentation by ChevronTexaco on crude oils with a high content of naphthenic acids
- Information by Seth Laboratories on Naphthenic acid corrosion
- Details regarding Kuwaitian heavy crudes and naphthenic acid corrosion
- Article regarding naphthenic acid removal
- Article regarding naphthenic acid species
- Article abstract regarding molecular origins of heavy crude oil interfacial activity mainly caused by Naphthenic acids
- Article about processes to remove Naphthenic acids
- Article about stabilisation of water-in-oil emulsions by naphthenic acids
- Spectometric Identification of Naphthenic Acids Isolated from Crude Oil
- Hydrogen flux and naphthenic acid corrosion