Muscone
| Muscone | ||
|---|---|---|
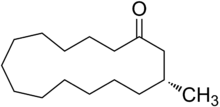 | ||
| IUPAC name (R)-3-methylcyclopentadecanone | ||
| Identifiers | ||
| CAS number | 541-91-3 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C16H30O | |
| Molar mass | 238.40 g/mol | |
| Density | 0.9221 g/cm3 | |
| Melting point | −15 °C; 5 °F; 258 K | |
| Boiling point | 328 °C; 622 °F; 601 K | |
| Hazards | ||
| NFPA 704 |
 1
1
1
| |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Muscone is an organic compound that is the primary contributor to the odor of musk.
The chemical structure of muscone was first elucidated by Lavoslav Ružička. It consists of a 15-membered ring ketone with one methyl substituent in the 3-position. It is an oily liquid that is found naturally as the (−)-enantiomer, but synthetic material is typically a racemate. It is very slightly soluble in water and miscible with alcohol.
Natural muscone is obtained from musk, a glandular secretion of the musk deer, which has been used in perfumery and medicine for thousands of years. Since obtaining natural musk requires killing the endangered animal, nearly all muscone used in perfumery today is synthetic. It has the characteristic smell of being "Musky". One asymmetric synthesis of (−)-muscone begins with commercially available (+)-citronellal, and forms the 15-membered ring via ring-closing metathesis:[1]
Muscone is now produced synthetically for use in perfumes and for scenting consumer products.
See also
References
- ↑ Kamat, V.P.; Hagiwara, H.; Katsumi, T.; Hoshi, T.; Suzuki, T.; Ando, M. (2000). "Ring Closing Metathesis Directed Synthesis of (R)-(−)-Muscone from (+)-Citronellal". Tetrahedron 56 (26): 4397–4403. doi:10.1016/S0040-4020(00)00333-1.
