Molybdate
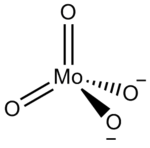
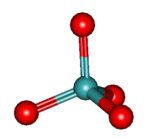
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state.The larger oxoanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates.[1] The discrete molybdenum oxoanions range in size from the simplest MoO42−, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO42−, Cr2O72−, Cr3O102− and Cr4O132− ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.[2]
Examples of molybdate anions
Examples of molybdate oxoanions are:
- discrete Mo2O72− in the tetrabutylammonium salt and polymeric Mo2O72− in the ammonium salt;[3]
- polymeric Mo3O102− in the ethylenediamine salt;[4]
- polymeric Mo4O132− in the potassium salt;[5]
- polymeric Mo5O162− in the anilinium, (C6H5NH3)+ salt;[6]
- discrete Mo6O192−(hexa-molybdate) in the tetramethylammonium salt;[7]
- discrete Mo7O246− in ammonium molybdate, (NH4)6Mo7O24.4H2O;[8]
- Mo8O264− in trimethylammonium salt.[1]
The naming of molybdates generally follows the convention of a prefix to show the number of Mo atoms present. For example, dimolybdate for 2 molybdenum atoms; trimolybdate for 3 molybdenum atoms, etc.. Sometimes the oxidation state is added as a suffix, such as in pentamolybdate(VI). The heptamolybdate ion, Mo7O24 6−, is often called "paramolybdate".
Structure of molybdate anions
The smaller anions, MoO42− and Mo2O72− feature tetrahedral centres. Mo2O72− can be considered to be two tetrahedra sharing a corner, i.e. with a single bridging O atom.[1] In the larger anions molybdenum is generally, but not exclusively, 6 coordinate with edges or vertices of the MoO6 octahedra being shared. The octahedra are distorted, typical M-O bond lengths are:
- in terminal non bridging M-O approximately 1.7 Å
- in bridging M-O-M units approximately 1.9 Å
The Mo8O264− anion contains both octahedral and tetrahedral molybdenum and can be isolated in 2 isomeric forms, alpha and beta.[2]
The hexamolybdate image below shows the coordination polyhedra. The heptamolybdate image shows the close packed nature of the oxygen atoms in the structure. The oxide ion has an ionic radius of 1.40 Å, molybdenum(VI) is much smaller, 0.59 Å.[1] There are strong similarities between the structures of the molybdates and the molybdenum oxides, (MoO3, MoO2 and the "crystallographic shear" oxides, Mo9O26 and Mo10O29) whose structures all contain close packed oxide ions.[9]


Equilibria in aqueous solution
When MoO3, molybdenum trioxide is dissolved in alkali solution the simple MoO42− anion is produced. As the pH is reduced the first species to be formed is the heptamolybdate rather than any of the smaller anions:
- 7 MoO42− + 8H+
 Mo7O246− + 4H2O [2]
Mo7O246− + 4H2O [2]
As the pH is decreased the octamolybdate is formed further anions with 8 and probably 16–18 Mo atoms are present:[1]
- Mo7O246− + 3H+
 Mo8O264− + 2H2O [2]
Mo8O264− + 2H2O [2]
A further decrease leads to anions with probably 16–18 Mo atoms. However careful manipulation of the pH and temperature coupled with very long precipitation times can cause compounds with ions that do not appear to be in solution to be precipitated.[1]
Peroxomolybdates
Many peroxomolybdates are known. They tend to form upon treatment of molybdate salts with hydrogen peroxide. Notable is including the monomer-dimer equilibrium [Mo2O3(O2)2(H2O)2]2-/ [Mo2O3(O2)4(H2O)2]2-. Also known but unstable is [Mo(O2)4]2- (see potassium tetraperoxochromate(V)). Some related compounds find use as oxidants in organic synthesis.[10]
Tetrathiomolybdate
The red tetrathiomolybdate anion results when molybdate solutions are treated with hydrogen sulfide:
- [NH4]2[MoO4] + 4 H2S → [NH4]2[MoS4] + 4 H2O
Like molybdate itself, [MoS4]2- undergoes condensation in the presence of acids, but these condensations are accompanied by redox processes.
Industrial uses
Catalysis
Molybdates are widely used in catalysis. In terms of scale, the largest consumer of molybdate is as a precursor to catalysts for hydrodesulfurization, the process by which sulfur is removed from petroleum. Bismuth molybdates, nominally of the composition Bi9PMo12O52, catalyzes ammoxidation of propylene to acrylonitrile. Ferric molybdates are used industrially to catalyze the oxidation of methanol to formaldehyde.[11]
Corrosion inhibitors
Sodium molybdate has been used in industrial water treatment as a corrosion inhibitor. It was initially thought that it would be a good replacement for chromate, when chromate was banned for toxicity. However, molybdate displays only moderate corrosion inhibition,[12] and is mainly used in high temperature closed-loop cooling circuits.[13]
Other
Nitrogen fixation requires molybdoenzymes in legumes (e.g., soybeans, acacia, etc.). For this reason, fertilizers often contain small amounts of molybdate salts. Coverage is typically less than a kilogram per acre. Molybdate chrome pigments are speciality but commercially available pigments.[11] Molybdate (usually in the form of potassium molybdate) is also used in the analytical colorimetric testing for the concentration of silica in solution, called the molybdenum blue method.[14] Additionally, it is used in the colorimetric determination of phosphate quantity in association with the dye malachite green.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ 2.0 2.1 2.2 2.3 Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ V. W. Day; M. F. Fredrich; W. G. Klemperer; W. Shum (1977). "Synthesis and characterization of the dimolybdate ion, Mo2O72−". Journal of the American Chemical Society 99 (18): 6146. doi:10.1021/ja00460a074.
- ↑ Guillou N.; Ferey G. (August 1997). "Hydrothermal Synthesis and Crystal Structure of Anhydrous Ethylenediamine Trimolybdate (C
2H
10N
2)[Mo
3O
10]". Journal of Solid State Chemistry 132 (1): 224–227(4). Bibcode:1997JSSCh.132..224G. doi:10.1006/jssc.1997.7502. - ↑ B. M. Gatehouse; P. Leverett (1971). "Crystal structure of potassium tetramolybdate, K
2Mo
4O
13, and its relationship to the structures of other univalent metal polymolybdates". J. Chem. Soc. A: 2107–2112. doi:10.1039/J19710002107. - ↑ W. Lasocha; H. Schenk (1997). "Crystal Structure of Anilinium Pentamolybdate from Powder Diffraction Data. The Solution of the Crystal Structure by Direct Methods Package POWSIM". J. Appl. Cryst. 30 (6): 909–913. doi:10.1107/S0021889897003105.
- ↑ S. Ghammami (2003). "The crystal and molecular structure of bis(tetramethylammonium) hexamolybdate(VI)". Cryst. Res. Technol. 38 (913): 913. doi:10.1002/crat.200310112.
- ↑ Howard T. Evans jr.; Bryan M. Gatehouse; Peter Leverett (1975). "Crystal structure of the heptamolybdate(VI)(paramolybdate) ion, [Mo7O24]6−, in the ammonium and potassium tetrahydrate salts". J. Chem. Soc., Dalton Trans. (6): 505–514. doi:10.1039/DT9750000505.
- ↑ Oxides: solid state chemistry W.H. McCarroll Encyclopedia of Inorganic Chemistry Ed. R. Bruce King, John Wiley and sons (1994)ISBN 0-471-93620-0
- ↑ Michael H. Dickman , Michael T. Pope "Peroxo and Superoxo Complexes of Chromium, Molybdenum, and Tungsten" Chem. Rev., 1994, vol. 94, pp 569–584.doi:10.1021/cr00027a002
- ↑ 11.0 11.1 Roger F. Sebenik et al. "Molybdenum and Molybdenum Compounds" in Ullmann's Encyclopedia of Chemical Technology 2005; Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_655
- ↑ http://www.gewater.com/handbook/cooling_water_systems/ch_31_open.jsp
- ↑ http://www.gewater.com/handbook/cooling_water_systems/ch_32_closed.jsp
- ↑ http://www.astm.org/Standards/D7126.htm