Mipomersen
| Systematic (IUPAC) name | |
|---|---|
| 2'-O-(2-methoxyethyl)-P-thioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiouridylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-deoxy-P-thioadenylyl-(3'→5')-2'-deoxy-Pthioguanylyl-(3'→5')P-thiothymidylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-2'-deoxy-P-thioguanylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-Pthioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-P-thioadenylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methylcytidine nonadecasodiumsalt | |
| Clinical data | |
| Trade names | Kynamro |
| Legal status | ? |
| Routes | Injection |
| Identifiers | |
| CAS number | 629167-92-6 |
| ATC code | C10AX11 |
| PubChem | CID 44564107 |
| UNII | 9GJ8S4GU0M |
| ChEMBL | CHEMBL502097 |
| Chemical data | |
| Formula | C230H305N67Na19O122P19S19 |
| Mol. mass | 7594.80 g/mol |
| SMILES
| |
| |
Mipomersen (previously ISIS 301012, trade name Kynamro) is a cholesterol-reducing drug candidate. It is an antisense therapeutic that targets the messenger RNA for apolipoprotein B.[1][2][3] It is administered as a weekly injection.
Structure
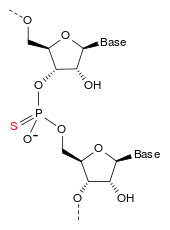
The compound is a 'second-generation' antisense oligonucleotide; the nucleotides are linked with phosphorothioate linkages rather than the phosphodiester linkages of RNA and DNA, and the sugar parts are deoxyribose in the middle part of the molecule and 2'-O-methoxyethyl-modified ribose at the two ends. These modifications make the drug resistant to degradation by nucleases, allowing it to be administered weekly. The drug accumulates in the liver, which is convenient since apolipoprotein B predominantly acts there.
The complete sequence is
G*-C*-C*-U*-C*-dA-dG-dT-dC-dT-dG-dmC-dT-dT-dmC-G*-C*-A*-C*-C*[d= 2'-deoxy,*= 2'-O-(2-methoxyethyl)]
with 3'→5' phosphorothioate linkages.[4]
Clinical development
The drug was discovered and developed to Phase 2 POC by ISIS Pharmaceuticals and subsequently licensed to Genzyme Corporation in an auction-style licensing bid. Isis earned an upfront payment of $325 million, with payments of a further $825 million if certain milestones are met. The licensing deal closed in June 2008.[5]
Mipomersen completed two separate phase-3 trials with two more Phase 3 trials to be presented in mid-2010.
The two completed Phase 3 trials were in patients with familial hypercholesterolemia (FH), both homozygous (ho)[6] and heterozygous (he).[7] FH is a genetic disorder that causes exceptionally high levels of low-density lipoprotein cholesterol. Both trials showed exceptional performance with the highest efficacy seen so far in those two patient populations, and with relatively low drop-out rates compared to other injectable drugs. Both of these trials also demonstrated the continued manageable safety and tolerability profile of mipomersen.[citation needed]
The remaining Phase 3 studies are due to be presented shortly (mid-2010) and include patients at high risk (HR) or who are severe hypercholesterolemic (SH).
August 2010: Third and fourth phase 3 trials were stated to have met primary and secondary endpoints.[8]
Genzyme plans to file for the first indication in the first half of 2011 in both the US and EU for hoFH & SH. Subsequently Genzyme plans on filing in the EU only for heFH as the FDA is requesting an outcome study be performed for any indication beyond the first.[citation needed]
Mipomersen is a synthetic molecule and launch quantities will be manufactured by Isis at their Carlsbad manufacturing facilities, and fill & finish will be performed at a third party facility.[citation needed]
In addition to the Phase 3 program, mipomersen has been tested in statin-intolerant patients in a small Phase 2 study.[9] Also, alternative dosing regimens of mipomersen are being tested in Phase 1 studies. The alternative dosing regimen studies are to address long term patient and physician convenience for administering the drug. The drug, if approved, will be provided in a small size needle syringe and can be self-injected by patients. The needle is only one gauge larger than that of insulin making it possibly acceptable and tolerable from the patient's perspective[citation needed] by decreasing the pain of injection. The inconvenience factor of injecting the medication, as opposed to swallowing a pill will likely factor in potentially treating high and moderate risk populations.
In January 2013, The United States Food and Drug Administration approved mipomersen for the treatment of homozygous familial hypercholesterolemia.[10][11]
References
- ↑ Merki E, Graham MJ, Mullick AE, et al. (August 2008). "Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice". Circulation 118 (7): 743–53. doi:10.1161/CIRCULATIONAHA.108.786822. PMID 18663084.
- ↑ El Harchaoui K, Akdim F, Stroes ES, Trip MD, Kastelein JJ (2008). "Current and future pharmacologic options for the management of patients unable to achieve low-density lipoprotein-cholesterol goals with statins". Am J Cardiovasc Drugs 8 (4): 233–42. doi:10.2165/00129784-200808040-00003. PMID 18690757.
- ↑ Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP (July 2008). "Antisense technology for the prevention or the treatment of cardiovascular disease: the next blockbuster?". Expert Opin Investig Drugs 17 (7): 969–72. doi:10.1517/13543784.17.7.969. PMID 18549334.
- ↑ Statement on a nonproprietary name adopted by the USAN council: Mipomersen sodium
- ↑ "Genzyme and Isis Complete Licensing of Mipomersen". 24 June 2008.
- ↑ ClinicalTrials.gov NCT00607373 Study to Assess the Safety and Efficacy of ISIS 301012 (Mipomersen) in Homozygous Familial Hypercholesterolemia (RADICHOL 1)
- ↑ ClinicalTrials.gov NCT00706849 Efficacy and Safety Study of ISIS 301012 (Mipomersen) as Add-on in Familial Hypercholesterolemic Subjects With Coronary Artery Disease (RADICHOL II)
- ↑ "Genzyme, Isis announce results of two mipomersen phase 3 studies".
- ↑ "Mipomersen shows large LDL reduction, but side effects an issue". theHeart.org. November 2011.
- ↑ Pollack, Andrew (29 January 2013) F.D.A. Approves Genetic Drug to Treat Rare Disease The New York Times, Retrieved 31 January 2013
- ↑ Staff (29 January 2013) FDA approves new orphan drug Kynamro to treat inherited cholesterol disorder U.S. Food and Drug Administration, Retrieved 31 January 2013
External links
| |||||||||||||||||||||||||||||||||||||||||||