Metopimazine
 | |
|---|---|
| Systematic (IUPAC) name | |
| 1-(3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl)piperidine-4-carboxamide | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | ? |
| Identifiers | |
| CAS number | 14008-44-7 |
| ATC code | A04AD05 |
| PubChem | CID 26388 |
| ChemSpider | 24584 |
| UNII | 238S75V9AV |
| ChEMBL | CHEMBL398615 |
| Chemical data | |
| Formula | C22H27N3O3S2 |
| Mol. mass | 445.6 g/mol |
| SMILES
| |
| |
| | |
Metopimazine (INN) is a phenothiazine antiemetic.
Synthesis
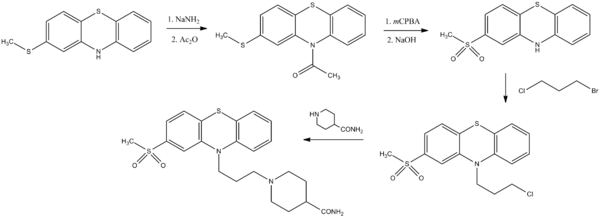
The reduced basicity of phenothiazine nitrogen requires that even acylation proceed via the anion. The amide () from the methyl thioether () can be prepared, for example, by sequential reaction with sodium amide and acetic anhydride. Oxidation of that intermediate with peracid proceeds preferentially on the more electron-rich alkyl thioether to give the sulfone; this affords the phenothiazine () on hydrolysis of the amide. Complex side chains are most conveniently incorporated in a stepwise fashion. The first step in the present sequence involves reactionof () as its anion with 1-bromo-3-chloropropane to give (). The use of that halide with alkylate piperidine-4-carboxamide () affords the antipsychotic agent metopimazine ().[1]
References
- ↑ Jacob, R. M.; Robert, J. G.; 1959, DE 1092476.